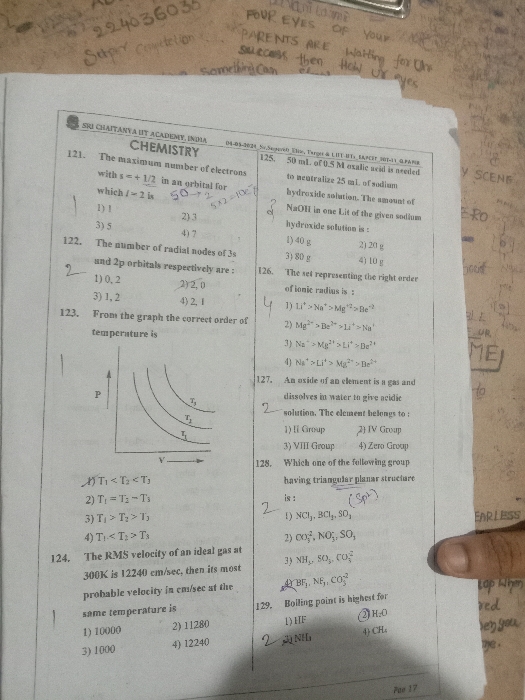
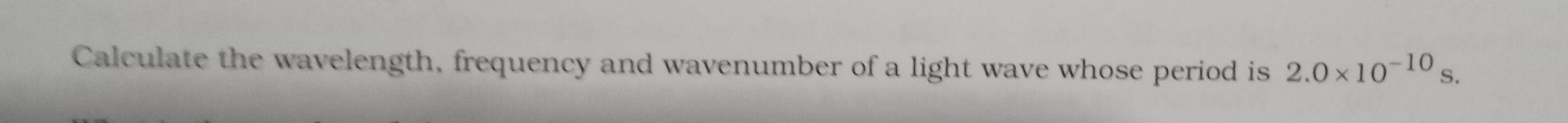JEE Class main Answered
Calculate the Ryderburg constant R if He+ ions are known to have the wavelength difference between the first (of the longest wavelength)lines of Balmer and Lyman series equal to 133.7 nm..Sir plz do calculations visible to me also..thanks
Asked by vishakhachandan026 | 19 May, 2019, 21:04: PM
Wave length of the first line of Balmer series
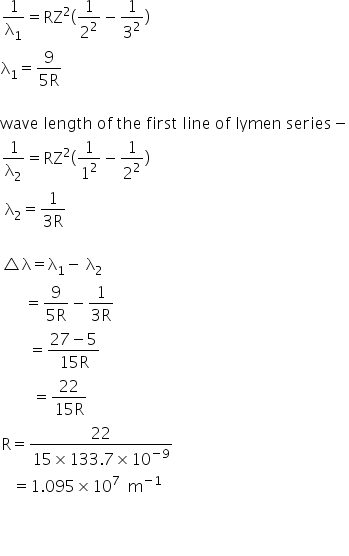
Answered by Ravi | 20 May, 2019, 14:55: PM
JEE main - Chemistry
Asked by radham6375 | 17 May, 2024, 20:13: PM
JEE main - Chemistry
Asked by 9079344910choudhary | 16 May, 2024, 18:52: PM
JEE main - Chemistry
Asked by purnendurai26 | 02 May, 2024, 18:34: PM
JEE main - Chemistry
Asked by cheekatiyogendra143 | 20 Apr, 2024, 11:16: AM
JEE main - Chemistry
Asked by jwhhebbb | 19 Apr, 2024, 13:21: PM
JEE main - Chemistry
Asked by adityadoodi3 | 05 Apr, 2024, 23:27: PM
JEE main - Chemistry
Asked by pratap62437 | 19 Feb, 2024, 12:48: PM
JEE main - Chemistry
Asked by sayushman087 | 01 Feb, 2024, 10:28: AM
JEE main - Chemistry
Asked by marthalamanoharreddy65 | 17 Dec, 2023, 10:26: AM