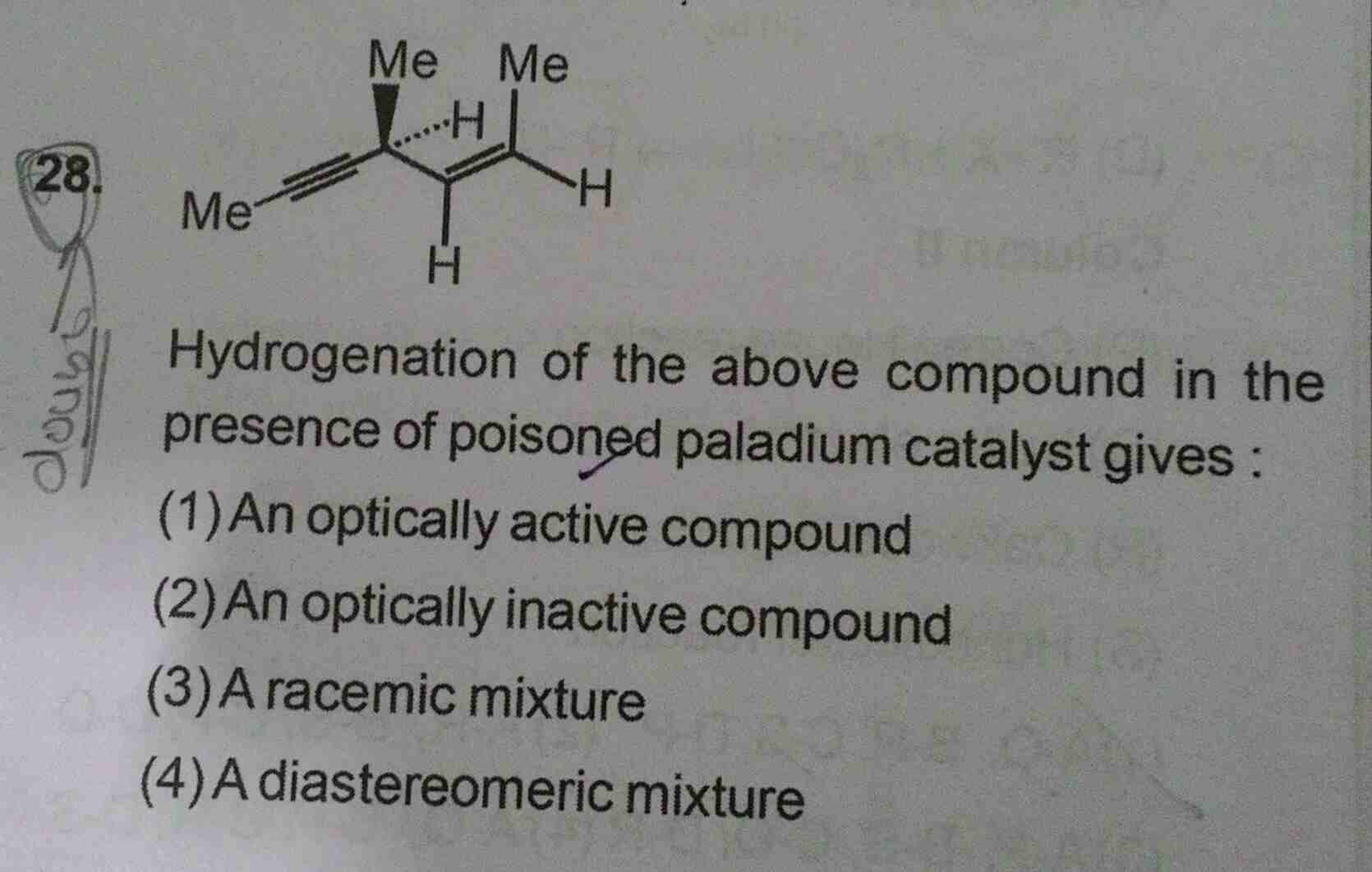
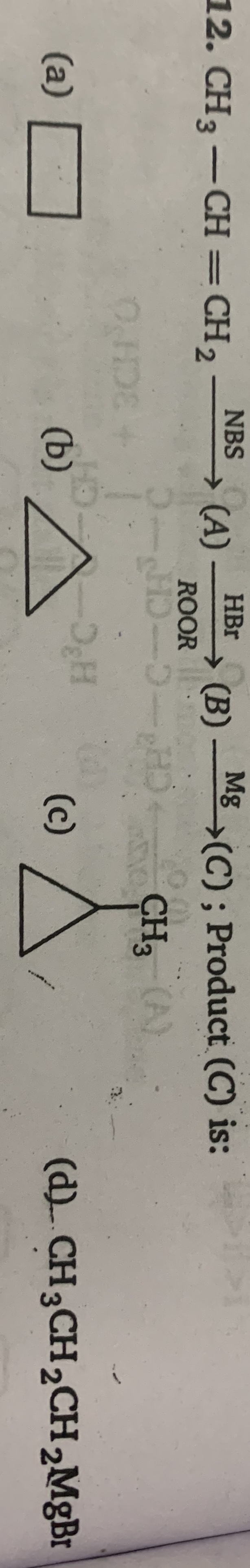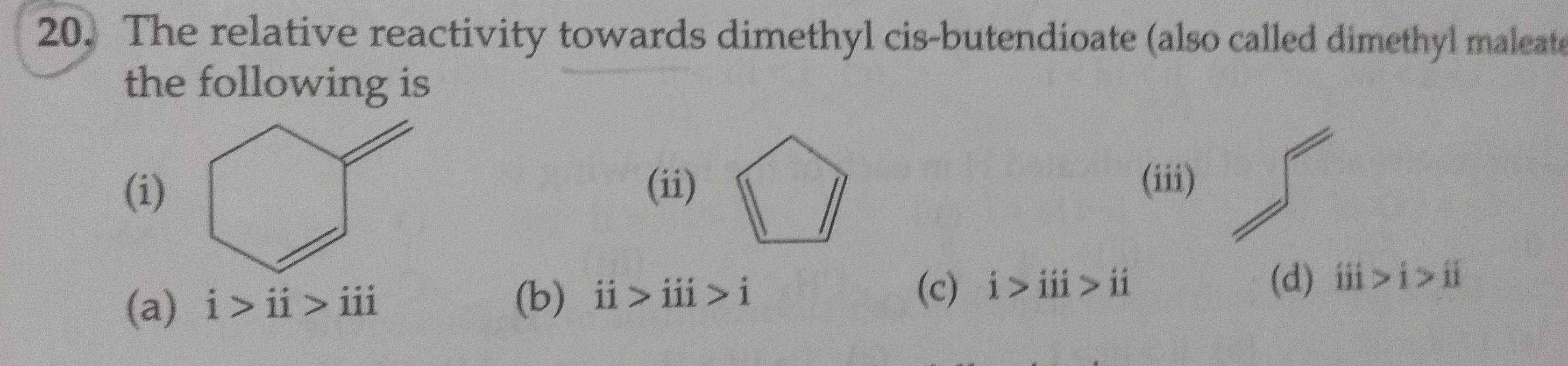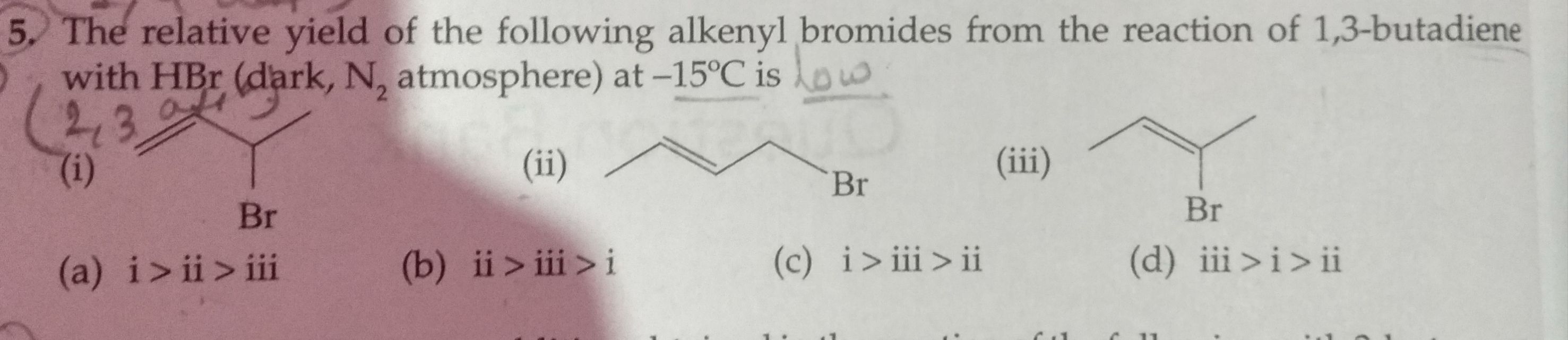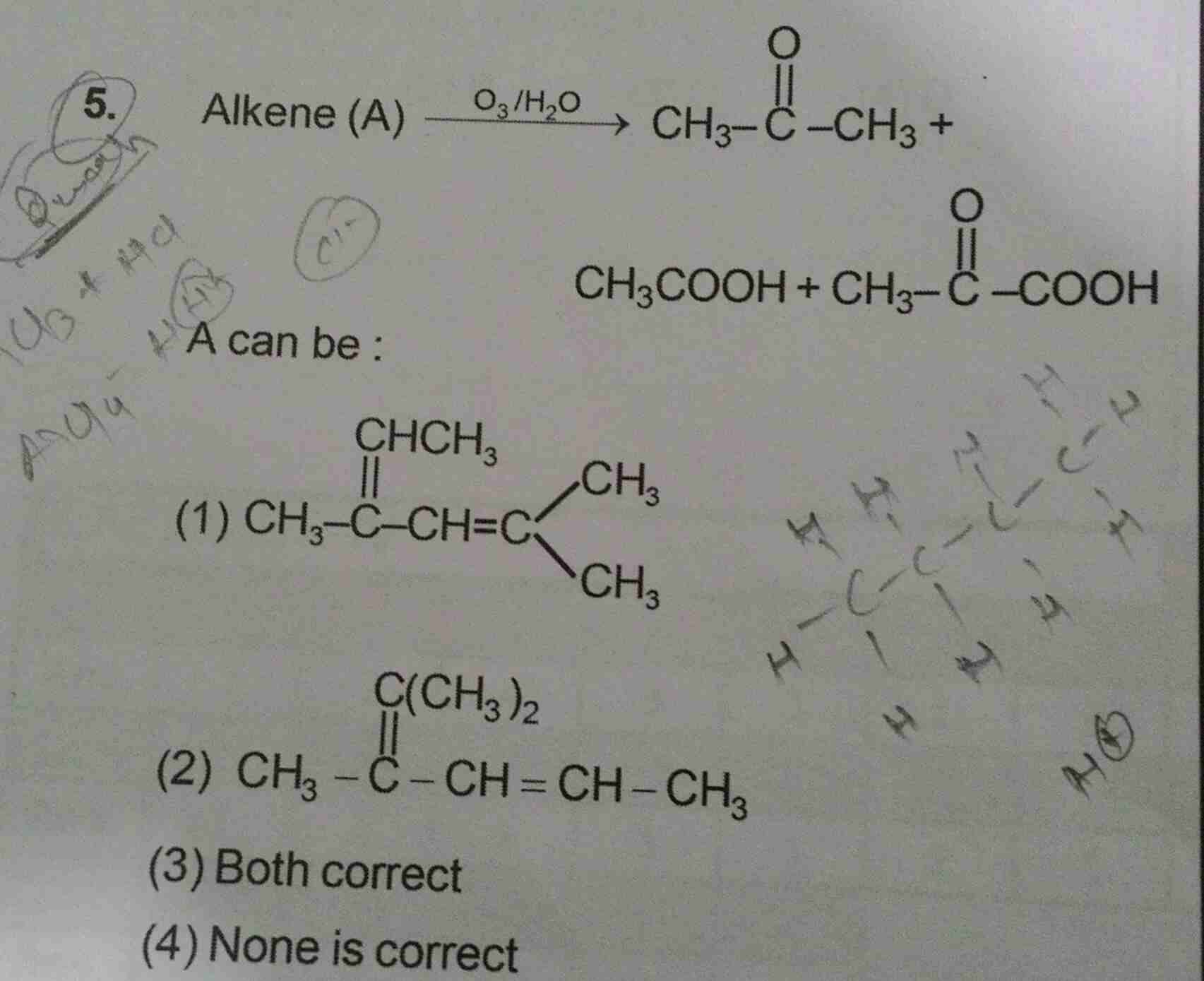NEET Class neet Answered
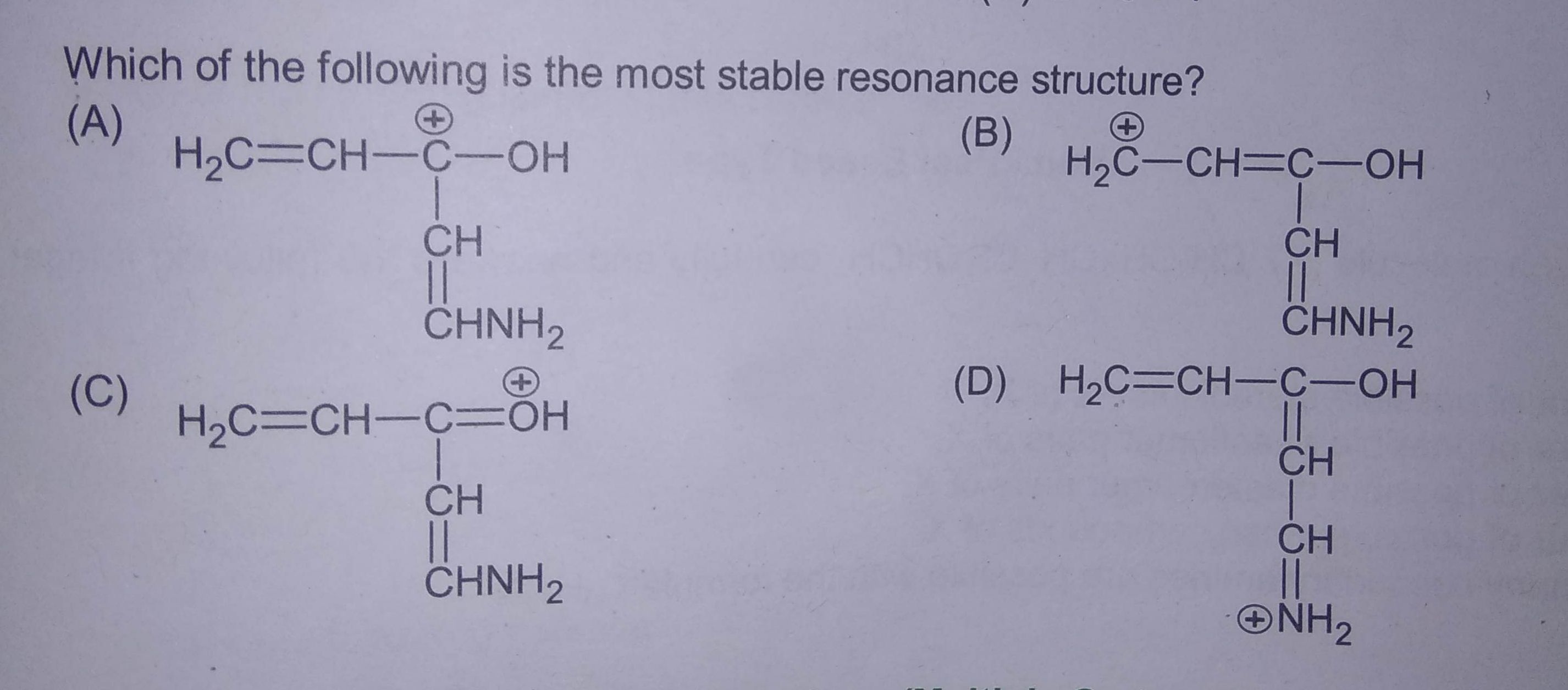
At high temperature Thermodynemically stable products are formed...plz Explain the answer properly...(it is given option b)
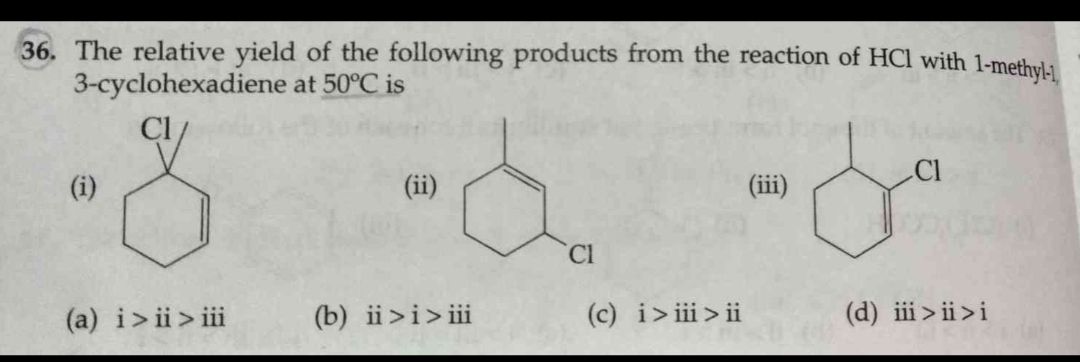
Asked by jhajuhi19 | 17 Feb, 2020, 01:36: AM
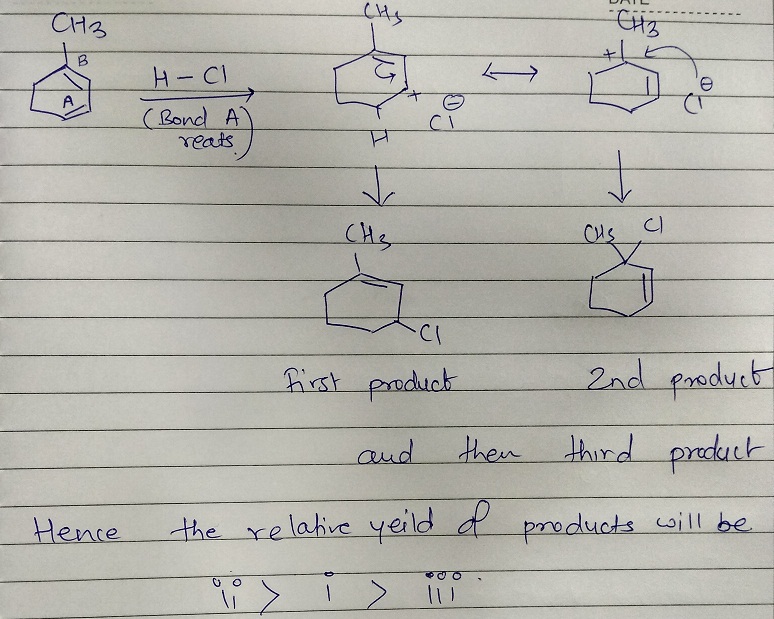
To obtained thermodynamically stable product this reaction needs more temperature.
Answered by Ramandeep | 17 Feb, 2020, 16:28: PM
NEET neet - Chemistry
Asked by nagendrakakumuri | 04 Nov, 2023, 22:11: PM
NEET neet - Chemistry
Asked by sulakshansnaik | 16 Dec, 2022, 17:47: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 22 Mar, 2020, 04:13: AM
NEET neet - Chemistry
Asked by vinaymprasad3 | 14 Mar, 2020, 14:40: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 17 Feb, 2020, 01:36: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 16 Feb, 2020, 02:20: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 15 Feb, 2020, 01:05: AM
NEET neet - Chemistry
Asked by Prashant DIGHE | 13 Feb, 2020, 21:03: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 13 Feb, 2020, 21:00: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 13 Feb, 2020, 20:59: PM