CBSE Class 10 Answered
answer required urgently
Asked by ardas1994 | 23 Jan, 2010, 08:36: PM
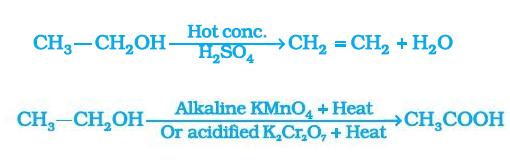
Ethanol when reacted with hot conc. sulphuric acid at 443 K, forms ethene .
Onon adding potassium permanganate to ethanol , the colour of the solution disappears because potassium permanganate os being consumed in the reaction to convert ethanol to ethanoic acid.
When excess of potassium permanganate is added, till ethanol is being converted to ethanoic acid, it remains as colourless. But when complete ethanol is converted, the potassium permangatanate is not being consumed and hence the left over potassium permanganate gives pink colour to the solution.
Answered by | 25 Jan, 2010, 10:21: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by parthmarch1 | 14 Dec, 2023, 08:27: PM
CBSE 10 - Chemistry
Asked by reetritu34 | 14 Dec, 2023, 07:54: AM
CBSE 10 - Chemistry
Asked by agarwalkrishnam98 | 01 Oct, 2023, 08:28: AM
CBSE 10 - Chemistry
Asked by asra964072 | 18 May, 2022, 10:03: PM
CBSE 10 - Chemistry
Asked by jainnikhil668 | 05 May, 2022, 02:00: PM
CBSE 10 - Chemistry
Asked by gsvjairam | 17 Apr, 2022, 11:32: AM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 10 Feb, 2022, 08:43: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:02: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 03:01: PM
CBSE 10 - Chemistry
Asked by sivaramaraju1000 | 21 Jan, 2022, 09:05: AM