CBSE Class 12-science Answered
a proton and alpha particle is accelerated through the same potential which one of the two has
1) greater value of de-Broglie wavelength associated with it?
2) less kinetic energy?
Asked by modi72879 | 25 Nov, 2017, 11:29: AM
A proton and an a-particle are accelerated through the same potential. Which one of the two has
(i) greater value of de-Broglie wavelength associated with it and
(ii) less kinetic energy?
Give reasons to justify your answer.
Solution:
1) Using de-Broglie wavelength formula, we already know that proton and alpha particle are accelerated through the same potential.
So, both their velocities are same.
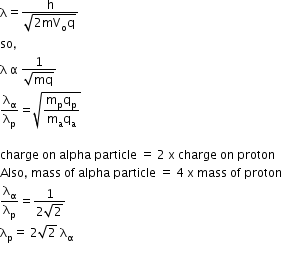
Therefore, proton has a greater de-Broglie wavelength than the alpha particle.
2) For same potential of acceleration, K.E is directly proportional to the 'q'
As, the charge of an alpha particle is more than the proton,
hence proton has a lesser value of Kinetic Energy (K.E)
Answered by Abhijeet Mishra | 25 Nov, 2017, 23:27: PM
Concept Videos
CBSE 12-science - Physics
Asked by mazhartahsildar143 | 07 May, 2020, 13:44: PM
CBSE 12-science - Physics
Asked by Hemanthrakshitha95 | 03 Mar, 2020, 14:55: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 08 Jan, 2019, 16:33: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 21 May, 2014, 09:18: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 21 May, 2014, 09:41: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 19 May, 2014, 16:38: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 21 May, 2014, 09:49: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM




