NEET Class neet Answered
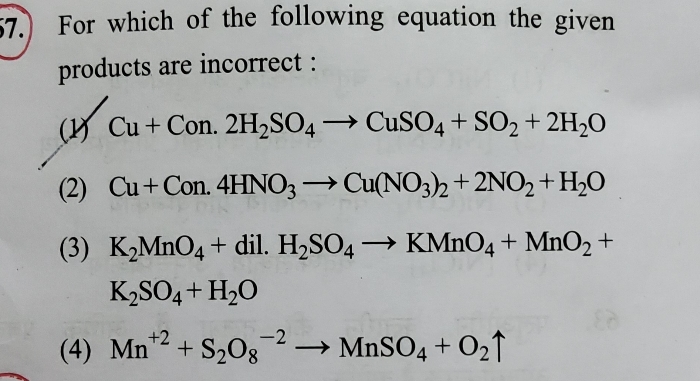
1 g mixture of CaCO3 and NaCl reacts completely with 100 ml of N/10 HCl. The % of CaCO3 in the mixture is-(1) 40%, (2)60%, (3) 50%, (4) 80%. Also mention which is to be taken as Solute, HCl or CaCO3??
Asked by patra04011965 | 07 Aug, 2019, 08:57: PM
Option (3) is correct: 50%
Given:
Mass of CaCO3 + NaCl = 0.1 gm
Volume of HCl = 100 ml
Normality of HCl = 0.1 N
Molarity of HCl = 0.1 M
Molar masss of CaCo3 = 100 g/mol
HCl reacts with CaCo3. The reaction is written as,
CaCo3 + 2HCl → CaCl2 + CO2 +H2O
1 mole of CaCo3 reacts with 2 moles of HCl
No. of moles of HCl is
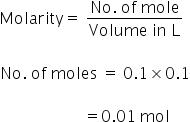
Now moles of CaCo3 can be calculated as,
Moles of CaCo3
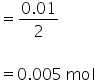
Mass of CaCo3 is calculated as,
No. of moles = 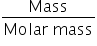
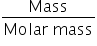
Mass of CaCo3 = No. of moles × Molar mass of CaCo3
= 0.005×100
= 0.5 gm
Percentage of CaCo3 = 0.5 × 100
= 50 %
Answered by Varsha | 08 Aug, 2019, 11:04: AM
Concept Videos
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by jetabanborthakur123 | 29 Mar, 2024, 07:05: PM
NEET neet - Chemistry
Asked by vaka.aruna1979 | 23 Mar, 2024, 04:18: AM
NEET neet - Chemistry
Asked by fathimahusna23042004 | 03 Mar, 2024, 08:56: AM
NEET neet - Chemistry
Asked by drkeshavkhandagle | 18 Jan, 2024, 08:10: PM
NEET neet - Chemistry
Asked by yogitakumawat | 21 Dec, 2023, 10:31: PM