Class 9 SELINA Solutions Chemistry Chapter 1: The Language of Chemistry
The Language of Chemistry Exercise Ex. 1(A)
Solution 1(a)
iii. The formula of a compound represents a molecule.
Solution 1(b)
iii. The correct formula of aluminium oxide is Al2O3.
Solution 1(c)
iv. The valency of nitrogen in nitrogen dioxide (NO2) is four.
Solution 2
|
Compound |
Formula (Ans) |
| a. Boric acid |
xvi. H3BO3 |
| b. Phosphoric acid |
xvii. H3PO4 |
| c. Nitrous acid |
xv. HNO2 |
| d. Nitric acid |
xiv. HNO3 |
| e. Sulphurous acid |
xiii. H2SO3 |
| f. Sulphuric acid |
xviii. H2SO4 |
| g. Hydrochloric acid |
xii. HCl |
| h. Silica (sand) |
ii. SiO2 |
| i. Caustic soda (sodium hydroxide) |
i. NaOH |
| j. Caustic potash (potassium hydroxide) |
iv. KOH |
| k. Washing soda (sodium carbonate) |
iii. Na2CO3 |
|
l. Baking soda (sodium bicarbonate) |
vi. NaHCO3 |
|
m. Lime stone (calcium carbonate) |
v. CaCO3 |
| n. Water |
viii. H2O |
| o. Hydrogen sulphide |
vii. H2S |
| p. Ammonia |
xi. NH3 |
| q. Phosphine |
ix. PH3 |
| r. Methane |
x. CH4 |
Solution 3
|
|
Acidic radical |
Basic radical |
|
SO4- |
Mg+ |
|
SO4- |
NH4+ |
|
SO4- |
Al3+ |
|
CO3- |
Zn2+ |
|
OH- |
Mg2+ |
Solution 4
|
|
Name |
Formula |
Valency |
|
a. |
Aluminate |
AlO2 |
-2 |
|
b. |
Chromate |
CrO4 |
-2 |
|
c. |
Aluminium |
Al |
+3 |
|
d. |
Cupric |
Cu |
+2 |
Solution 5
a. H - Hydrogen atom
b. H2 - Dihydrogen or hydrogen gas
c. 2H - Two hydrogen atoms
d. 2H2 - Two dihydrogen molecule
Solution 6
Chemical names of compounds:
- Ca3(PO4)2 - Calcium phosphate
- K2CO3 - Potassium carbonate
- K2MnO4 - Potassium manganate
- Mn3(BO3)2 - Manganese (II) borate
- Mg(HCO3)2 - Magnesium hydrogen carbonate
- Na4Fe(CN)6 - Sodium ferrocyanide
- Ba(ClO3)2 - Barium chlorate
- Ag2SO3 - Silver sulphite
- (CH3COO)2Pb - Lead acetate
- Na2SiO3 - Sodium silicate
Solution 7
Valencies of aluminium, ammonium and zinc are 3, 1 and 2, respectively.
The valency of sulphate is 2.
Hence, chemical formulae of the sulphates of aluminium, ammonium and zinc are Al2(SO4)3, (NH4)2SO4 and ZnSO4.
Solution 8
Formula of the compound = A2B3
Solution 9
- KClO- Potassium hypochlorite
- KClO2- Potassium chlorite
- KClO3- Potassium chlorate
- KClO4- Potassium perchlorate
Solution 10
The valency of metal M is 3. So, the formulae are as follows:
- Chloride - MCl3
- Oxide - M2O3
- Phosphate - M(PO4)
- Acetate - M(CH3COO)3
Solution 11
A symbol is the short form which stands for the atom of a specific element or the abbreviations used for the names of elements.
i. It represents a specific element.
ii. It represents one atom of an element.
iii. A symbol represents how many atoms are present in its one gram (gm) atom.
iv. It represents the number of times an atom is heavier than one atomic mass unit (amu) taken as a standard.
Solution 12
In most cases, the first letter of the name of the element is taken as the symbol for that element and written in capitals (e.g. for sulphur, we use the symbol S). In cases where the first letter has already been adopted, we use a symbol derived from the Latin name (e.g. for sodium/Natrium, we use the symbol Na). In some cases, we use the initial letter in capital together with a small letter from its name (e.g. for silicon, we use the symbol Si).
Solution 13
The full form of IUPAC is International Union of Pure and Applied Chemistry.
Names of the elements:
Au - Gold
Pb - Lead
Sn - Tin
Hg - Mercury
Solution 14
Co stands for Cobalt. If we write CO, then it would mean that it is a compound containing two non-metal ions, i.e. carbon and oxygen, which forms carbon monoxide gas.
Solution 15
The number of atoms of an element that join together to form a molecule of that element is known as its atomicity.
Diatomic molecules: H2, O2, N2, Cl2
Solution 16(a)
- Valency of Na is +1 because it can lose one electron.
- Valency of O is -2 because it can accept two electrons.
Variable valency: It is the combining capacity of an element in which the metal loses more electrons from a shell next to a valence shell in addition to electrons of the valence shell.
Solution 16(b)
If an element exhibits two different positive valencies, then
i. for the lower valency, use the suffix -OUS at the end of the name of the metal
ii. for the higher valency, use the suffix -IC at the end of the name of the metal.
Example:
|
Element |
Lower valency |
Higher valency |
|
Ferrum (Iron) |
Ferrous (Fe2+) |
Ferric (Fe3+) |
Solution 17
- Chemical formula: The chemical formula of a substance (element or compound) is a symbolic representation of the actual number of atoms present in one molecule of that substance.
- Significance of the molecular formula:
i. It represents both molecule and molecular mass of the compound.
ii. It represents the respective number of different atoms present in one molecule of the compound.
iii. It represents the ratios of the respective masses of the elements present in the compound.e.g. Hg2O 1. Hg1+O2-
2.
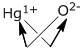
3. Hg2O
Solution 18(a)
Acid radical: The electronegative or negatively charged radical is called an acid radical.
Examples: Cl-, O2-
Solution 18(b)
Basic radical: The electropositive or positively charged radical is called a basic radical.
Examples: K+, Na+
Solution 19
|
Compounds |
Acidic radical |
Basic radical |
Chemical formulae |
| Barium sulphate |
SO42- |
Ba2+ |
BaSO4 |
| Bismuth nitrate |
NO3- |
Bi3+ |
Bi(NO3)3 |
| Calcium bromide |
Br- |
Ca2+ |
CaBr2 |
| Ferrous sulphide |
S2- |
Fe2+ |
FeS |
| Chromium sulphate |
SO42- |
Cr3+ |
Cr2(SO4)3 |
| Calcium silicate |
SiO42- |
Ca2+ |
Ca2SiO4 |
| Stannic oxide |
O2- |
Sn2+ |
SnO2 |
| Sodium zincate |
ZnO2- |
Na1+ |
Na2ZnO2 |
| Magnesium phosphate |
(PO4)3- |
Mg2+ |
Mg3(PO4)2 |
| Sodium thiosulphate |
(S2O3)2- |
Na1+ |
Na2S2O3 |
| Stannic phosphate |
(PO4)3- |
Sn4+ |
Sn3(PO4)4 |
| Nickel bisulphate |
HSO41- |
Ni3+ |
Ni(HSO4)3 |
| Potassium manganate |
MnO42- |
K1+ |
K2MnO4 |
| Potassium ferrocyanide | [Fe(CN)6]4- | K1+ | K4[Fe(CN)6] |
Solution 20
- Sodium sulphate - Na2SO4
There are two sodium atoms, one sulphur atom and four oxygen atoms.
- Quick lime - CaO
There is one calcium atom and one oxygen atom.
- Baking soda - NaHCO3
There is one sodium, carbon and hydrogen atom and three oxygen atoms.
- Ammonia - NH3
There is one nitrogen atom and three hydrogen atoms.
- Ammonium dichromate - (NH4)Cr2O7
There two ammonium atoms, two chromium atoms and seven oxygen atoms.
The Language of Chemistry Exercise Ex. 1(B)
Solution 1
A chemical equation is the symbolic representation of a chemical reaction using the symbols and formulae of the substances involved in the reaction.
A chemical equation needs to be balanced because a chemical reaction is just a rearrangement of atoms.
Atoms themselves are neither created nor destroyed during the course of a chemical reaction.
The chemical equation needs to be balanced to follow the law of conservation of mass.
Solution 2
A solid metal zinc reacts with hydrochloric acid in the aqueous state to produce zinc chloride in the aqueous state and hydrogen gas.
Solution 3
- The chemical equation given in question 2 does not give the time taken for the completion of the reaction.
- Also, it does not give information about whether heat is absorbed or evolved during the reaction.
Solution 4
- C + O2→ CO2
- N2 + O2→ 2NO
- 3Ca + N2→ Ca3N2
- CaO + CO2→ CaCO3
- Mg + H2SO4→ MgSO4 + H2↑
- Na + H2O → NaOH + H2↑
Solution 5
Balanced chemical equations:
- 3Fe + 4H2O → Fe3O4 + 4H2
- 3Ca + N2 → Ca3N2
- Zn + 2KOH → K2ZnO2 + H2
- Fe2O3 + 3CO → 2Fe + 3CO2
- 3PbO + 2NH3 → 3Pb + 3H2O + N2
- 2Pb3O4 → 6PbO + O2
- 2PbS + 3O2 → 2PbO + 2SO2
- S + 2H2SO4 → 3SO2 + 2H2O
- S + 6HNO3 → H2SO4 + 6NO2 + 2H2O
- MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
- C + 2H2SO4 → CO2 + H2O + SO2
- 2KOH + Cl2 → KCl + KClO + H2O
- 2NO2 + H2O → HNO2 + HNO3
- Pb3O4 + 8HCl → 3PbCl2 + 4H2O + Cl2
- 2H2O + 2Cl2 → 4HCl + O2
- 2NaHCO3 → Na2CO3 + H2O + CO2
- 2HNO3 + H2S → 2NO2 + 2H2O + S
- P + 5HNO3 → 5NO2 + H2O + H3PO4
- Zn + 4HNO3 → Zn(NO3)2 + 2H2O + 2NO2
The Language of Chemistry Exercise Ex. 1(C)
Solution A. 1
iii. Berzelius
Solution A. 2
i. One
Solution A. 3
iii. Fe2(SO4)3
Solution A. 4
i. 1:8
Solution A. 5
ii. Ca(HCO3)2
Solution A. 6
Correct option - (iii): C, Ca, Cu, Cd
Solution:
The correct atomic symbols for carbon, calcium, copper and cadmium is C, Ca, Cu and Cd.
Solution A. 7
Correct option - (iii): Mercury
Solution:
Hydraygyrum is the Latin name of Mercury.
Solution A. 8
Correct option - (iv): 2 and 3
Solution:
Solution A. 9
Correct option - (ii): 2
Solution:
Calcium is divalent, hence it will bond with two molecules of phosphate anion.
Solution A. 10
Correct option - (iii): 4
Solution:
Carbon is tetravalent.
Solution B. 1
- Dalton used symbol [O] for oxygen,[H] for hydrogen.
- Symbol represents gram atom(s) of an element.
- Symbolic expression for a molecule is called molecular formula.
- Sodium chloride has two radicals. Sodium is a basic radical, while chloride is an acid radical.
- Valency of phosphorus in PCl3, is 3 and in PCl5 is 5.
- Valency of iron in FeCl2 is 2 and in FeCl3 it is 3.
- Formula of iron (III) carbonate is Fe2[CO3]3.
Solution B. 2
|
Acid Radicals
Basic Radicals |
Chloride |
Nitrate |
Sulphate |
Carbonate |
Hydroxide |
Phosphate |
|
Magnesium |
MgCl2 |
Mg(NO3)2 |
MgSO4 |
MgCO3 |
Mg(OH)2 |
Mg3(PO4)2 |
|
Sodium |
NaCl |
NaNO3 |
Na2SO4 |
Na2CO3 |
NaOH |
Na3PO4 |
|
Zinc |
ZnCl2 |
Zn(NO3)2 |
Zn(SO4)2 |
ZnCO3 |
Zn(OH)2 |
Zn3(PO4)2 |
|
Silver |
AgCl |
AgNO3 |
Ag2SO4 |
AgCO3 |
AgOH |
Ag3PO4 |
|
Ammonium |
NH4Cl |
NH4NO3 |
(NH4)2SO4 |
(NH4)2CO3 |
NH4OH |
(NH4)3PO4 |
|
Calcium |
CaCl2 |
CaCO3 |
CaSO4 |
CaCO3 |
Ca(OH)2 |
Ca3(PO4)2 |
|
Iron (II) |
FeCl2 |
Fe(NO3)2 |
FeSO4 |
FeCO3 |
Fe(OH)2 |
Fe3(PO4)2 |
|
Potassium |
KCl |
KNO3 |
K2SO4 |
K2CO3 |
KOH |
K3PO4 |
Solution B. 3
The correct statements are -
- The number of elements present in a molecule is represented by molecular formula.
- The molecular formula of water is H
 O. H
O. H O
O is the molecular formula of hydrogen peroxide.
is the molecular formula of hydrogen peroxide. - A molecule of sulfur is not monoatomic. It consists of 8 atoms.
- Co represents cobalt and CO represents carbon monoxide.
- The formula of iron (III) oxide is Fe2O3.
Solution B. 4
The empirical formula is:
- Benzene (C6H6) = CH
- Glucose (C6H12O6) = CH6O
- Acetylene (C2H2) = CH
- Acetic acid (CH3COOH) = C2H2O
Solution C. 1(a)
Valency of fluorine in CaF2 is -1.
Solution C. 1(b)
Valency of sulphur in SF6 is -6.
Solution C. 1(c)
Valency of phosphorus in PH3 is +3.
Solution C. 1(d)
Valency of carbon in CH4 is +4.
Solution C. 1(e)
Valency of Manganese in MnO2 is +4.
Solution C. 1(f)
Valency of Copper in Cu2O is +1
Solution C. 1(g)
Valency of Magnesium in Mg3N2 is +2
Solution C. 1(h)
Valency of nitrogen in the given compounds:
- N2O3 = N is +3
- N2O5 = N is +5
- NO2 = N is +4
- NO = N is +2
Solution C. 2
According to the law of conservation of mass, 'matter can neither be created nor can it be destroyed'. This is possible only if the total number of atoms on the reactants side is equal to the total number of atoms on the products side. Thus, a chemical reaction should always be balanced.
e.g. KNO3 → KNO2 + O2
In this equation, the number of atoms on both sides is not the same, and the equation is not balanced.
The balanced form of this equation is
2KNO3 → 2KNO2 + O2
Solution C. 3
Atomic mass unit (amu) is equal to one-twelfth the mass of an atom of carbon-12 (atomic mass of carbon taken as 12).
Solution C. 4(a)
2NaOH + H2SO4 → Na2SO4 + 2H2O
Solution C. 4(b)
2KHCO3 + H2SO4 → K2SO4 + 2CO2 + 2H2O
Solution C. 4(c)
Fe + H2SO4 → FeSO4 + H2
Solution C. 4(d)
Cl2 + SO2 + 2H2O → H2SO4 + 2HCl
Solution C. 4(e)
2AgNO3 → 2Ag + 2NO2 + O2
Solution C. 4(f)
3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O
Solution C. 4(g)
![]()
Solution C. 4(h)
BaCl2 + H2SO4 → BaSO4 + 2HCl
Solution C. 4(i)
2ZnS + 3O2 → 2ZnO + 2SO2
Solution C. 4(j)
Al4C3 + 12H2O → 4Al(OH)3 + 3CH4
Solution C. 4(k)
4FeS2 + 11O2 → 2Fe2O3 + 8SO2
Solution C. 4(l)
2KMnO4 + HCl → 2KCl + 2MnCl2 + 5Cl2 + 8H2O
Solution C. 4(m)
Al2(SO4)3 + 8NaOH → 3Na2SO4 + 2NaAlO2 + 4H2O
Solution C. 4(n)
2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2
Solution C. 4(o)
2K2Cr2O7 + 8H2SO4 → 2K2SO4 + 2Cr2(SO4)3 + 8H2O + 3O2
Solution C. 4(p)
K2Cr2O7 + 14HCl → 2KCl + 2CrCl3 + 7H2O + 3Cl2
Solution C. 4(q)
S + HNO3 → H2SO4 + NO2 + H2O
Solution C. 4(r)
2NaCl + MnO2 + 3H2SO4 → 2NaHSO4 + MnSO4 + 2H2O + Cl2
Solution D. 1
a. NaCl + AgNO3 → NaNO3 + AgCl↓
b. It is a balanced equation.
c. Weights of reactants: NaCl - 58.44, AgNO3 - 169.87
Weights of products: NaNO3 - 84.99, AgCl - 143.32
NaCl + AgNO3 → NaNO3 + AgCl
(23+35.5) + (108+14+48) → (23+14+48) + (108+35.5)
58.5 + 170 → 85 + 143.5
228.5 g → 228.5 g
d. Law of conservation of mass: Matter is neither created nor destroyed in the course of a chemical reaction.
Solution D. 2(a)
This equation conveys the following information:
- The actual result of a chemical change.
- Substances take part in a reaction, and substances are formed as a result of the reaction.
- Here, one molecule of zinc and one molecule of sulphuric acid react to give one molecule of zinc sulphate and one molecule of hydrogen.
- Composition of respective molecules, i.e. one molecule of sulphuric acid contains two atoms of hydrogen, one atom of sulphur and four atoms of oxygen.
- Relative molecular masses of different substances, i.e. molecular mass of
Zn = 65
H2SO4 = (2+32+64) = 98
ZnSO4 = (65+32+64) = 161
H2 = 2
- 22.4 litres of hydrogen are formed at STP.
Solution D. 2(b)
This equation conveys the following information:
- Magnesium reacts with hydrochloric acid to form magnesium chloride and hydrogen gas.
- 24 g of magnesium reacts with 2(1 + 35.5) = 73 g of hydrochloric acid to produce (24 + 71), i.e. 95 g of magnesium chloride.
- Hydrogen produced at STP is 22.4 litres.
Solution D. 3(a)
A polyatomic ion is a charged ion composed of two or more covalently bounded atoms. Examples: Carbonate (CO32-) and sulphate (SO42-)
Solution D. 3(b)
Fundamental laws which are involved in every equation:
- A chemical equation consists of formulae of reactants connected by a plus sign (+) and arrow (→) followed by the formulae of products connected by the plus sign (+).
- The sign of an arrow (→) is to read 'to form'. It also shows the direction in which the reaction is predominant.
The fundamental law followed by every equation is 'Law of Conservation of Mass'.
Solution D. 4
The symbolic representation of a molecule is known as formula or molecular formula.
A molecular formula also known as chemical formula employs symbols to denote the molecule of an element or of a compound.
• The chemical formula of a compound provides information about the types of atoms that make up the compound, as well as the ratio in which they are combined.
• The elements that make up the compound: The chemical symbols of the elements present in the compound are used to write the formula.
For example,
The chemical formula H2O tells us that the compound is made up of two hydrogen atoms (H) and one oxygen atom (O).
• A molecule of an element may contain one or more atoms of it.
For example,
A molecule of elements hydrogen, oxygen, nitrogen, chlorine, bromine and iodine, contains two atoms and are written as H2, O2, N2, Cl2, Br2 and I2 respectively.
• In case of a compound, the molecule containing different atoms united in a certain fixed ratio is represented by placing symbols of the elements present in it side by side indicating their numbers written in subscripts.
For example,
NH4Cl represents one molecule of ammonium chloride containing one atom of nitrogen, four atoms of hydrogen and one atom of chlorine.
Na2CO3 denotes one molecule of sodium carbonate which contains two atoms of sodium, one atom of carbon and three atoms of oxygen.
2H2O represents two molecules of water i.e. dihydrogen oxide, each molecule containing two atoms of hydrogen and one atom of oxygen.
Thus, by looking at a formula, we understand the ratio in which the different atoms are united to form the molecule.
Solution D. 5
The molecular formula of a compound has quantitative significance. It represents:
(i) Both the molecule and the molecular mass of the compound.
(ii) The respective numbers of different atoms present in one molecule of the compound.
(iii) The ratios of the respective masses of the elements present in the compound.
For example, the formula CO2 means that:
(i) The molecular formula of carbon dioxide is CO2.
(ii) Each molecule contains one carbon atom joined by chemical bonds with two oxygen atoms.
(iii) The molecular mass of carbon dioxide is 44, given that the atomic mass of carbon is 12 and that of oxygen is 16.
Solution D. 6
A radical is an atom or a group of atoms of the same or of different elements that behaves as a single unit with a positive or negative charge.
A radical is called a simple radical when it is an atom only like sodium (Na+) and magnesium (Mg2+). It is known as a compound radical when it is made up of a group of two or more different atoms (polyatomic) like sulphate (SO42-) which is made up of one sulphur atom and four oxygen atoms.
A radical with positive charge is a cation, e.g., NH4+ (ammonium ion), Na+ (sodium ion) and a radical with negative charge is an anion, e.g. Cl⁻ (chloride), CO32- (carbonate).An acid reacts with a base to produce salt and water as a result of neutralization.
Potassium hydroxide + Hydrochloric acid → Potassium chloride + Water
(Base) (Acid) (Salt)
In the formation of potassium chloride, the potassium radical has been contributed by the base potassium hydroxide and is therefore called a basic radical while the chloride radical has been contributed by hydrochloric acid and is, therefore, termed an acid radical.
Solution D. 7
|
Metal |
Valency |
Name of Compound Formed |
Formula |
|
Iron |
2 3 |
Ferrous [Iron(II)oxide] Ferric [Iron(III)oxide] |
FeO Fe2O3 |
|
Copper |
1 2 |
Cuprous [Copper(I)] oxide Cupric [Copper(II)] oxide |
Cu2O CuO |
|
Mercury |
1 2 |
Mercurous [Mercury (I)] oxide Mercuric [Mercury(II)] oxide |
Hg2O HgO |
|
Lead |
2 4 |
Plumbous [Lead (II)] oxide Plumbic [Lead (IV)] oxide |
PbO PbO2 |
Solution E. 1
(a) X and Y
Valence shell electrons in X and Y are 3 and 7 respectively.
3 × X = (8-7)×Y
By rearranging the equation,
X/Y = 1/3
Same number of electrons has to be shared/donated.
Hence, the chemical formula is XY3.
(b) X and Z
Valence shell electrons in X and Z are 3 and 6 respectively.
Same number of electrons has to be shared/donated.
3 × X = (8-6) × Y
X/Y = 2/3
Hence, the chemical formula of the compound is X2Y3.
Solution E. 2
(a)
The number of carbon atoms = 6
The number of hydrogen atoms = 12
(b) Molecular formula = C6H12
Empirical formula = CH6
(c) Percentage composition of all the elements present in the compound:
Relative atomic mass of C6H12 = (6×12)+(12×1) = 84 amu
% of carbon in the compound
= (atomic mass of carbon / atomic mass of molecule) × 100 = (72/84)×100 = 85.7% is the percentage of carbon atoms in the compound.
% of hydrogen in the compound
= (atomic mass of hydrogen / atomic mass of molecule) × 100 = (12/84) × 100 = 14.3% is the percentage of hydrogen atoms in the compound.
Solution F. 1(a)
Molecular mass of Na2SO4.10H2O
= (2⨯23) + 32 +(4⨯16)+(10⨯18)
= 46 + 32 + 64 + 180
= 322
Solution F. 1(b)
Molecular mass of (NH4)2CO3
= (2 × 14) + (8 × 1) + 12 + (3 × 16)
= 28 + 8 + 12 + 48
= 96
Solution F. 1(c)
Molecular mass of (NH2)2CO
= (14 × 2) + (4 × 1) + 12 + 16
= 28 + 4 + 12 + 16
= 60
Solution F. 1(d)
Molecular mass of Mg3N2
= (3 × 24) + (2 × 14)
= 72 + 28
= 100
Solution F. 2
- CHCl3 = (C)12 + (H)1+ (3Cl)3 x 35.5 = 119.5
- (NH4)2Cr2O7 = (2N)28 + (8H)8 + (2Cr)2 x 51.9+ (7O)7 x 16 = 252
- CuSO4.5H2O = (Cu)63.5 + (S)32 + (4O)64 + (5H2O)5 x 18 = 249.5
- (NH4)2SO4 = (2N)28 + (8H)8 + (S)32 + (4O)64 = 132
- CH3COONa = (C)12 + (3H)3 + (C)12 + (2O)32 + (Na)23 = 82
- Potassium chlorate KClO3 = (K)39 + (Cl)35.5 + (3O)48 = 122.5
- Ammonium chloroplatinate, (NH4)2PtCl6 = (2N)28 + (8H)8 + (Pt)195 + (6Cl)35.5 x 6 = 444
Solution F. 3
Given,
Mass of epsom salt, MgSO4.7H2O.= 246.36 g/mole
Mass of water = 18 g/mole
Mass of 7H2O = 7⨯18 = 126 g/mole
Now we have to calculate the percentage mass of water in the epsom salt.
% mass of water = ![]()
Now put all the given values in this formula, we get the percentage mass of water in the epsom salt.
% mass of water = ![]()
Therefore, the percentage mass of water in the epsom salt is, 51.14 %
Solution F. 4
- Calcium hydrogen phosphate Ca(H2PO4)2
Molecular mass of Ca(H2PO4)2 = 234 g/mole
Atomic weight of P = 31
% of P in Ca(H2PO4)2 = ![]()
= 26.49 % of P
- Calcium phosphate Ca3(PO4)2
Molecular mass of Ca3(PO4)2 = 310.17 g/mole
Atomic weight of P = 31
% of P in Ca(H2PO4)2 = ![]() = 19.98 % of P
= 19.98 % of P
Solution F. 5
Molecular mass of KClO3 = 122.5 g
% of K = 39 /122.5 = 31.8%
% of Cl = 35.5/122.5 = 28.98%
% of O = 3 × 16/122.5 = 39.18%
Solution F. 6
Urea has the formula NH2CONH2, i.e. CON2H4
Molecular mass of urea = 60 g/mol
Atomic mass of C = 12 and number of atoms = 1 i.e. 12 g/mol of urea
Thus the % of C = 12/60 × 100 = 20 %

