Class 10 SELINA Solutions Physics Chapter 6 - Spectrum
Spectrum Exercise Ex. 6A
Solution A.1
(d) Both deviation and dispersion.
Hint: When a white light ray falls on the first surface of a prism, light rays of different colours due to their different speeds in glass get refracted (or deviated) through different angles. Thus, the dispersion of white light into its constituent colours takes place at the first surface of prism.
Solution A.2
(d) All of the above
All the given statements are true.
(a) The dispersion of white light occurs at the first surface of the prism.
(b) The deviation of light rays occurs at both the surfaces of the prism.
(c) The prism does not produce colours, but it only splits the various colours present in the white light.
So, the correct option is (d).
Solution A.3
(c) 400 nm to 800 nm
The wavelength range for white light is 400 nm to 800 nm or 4000 Å to 8000 Å.
Solution A.4
(a) Violet < Green < Red
We know the mnemonic VIBGYOR.
In VIBGYOR, the wavelength of the colours is listed in increasing order.
Thus, option (a) is correct.
Solution A.5
(c) Red
Hint: The angle of deviation decreases with the increase in wavelength of light for a given angle of incidence. Since the red light has greatest wavelength, it gets deviated the least and is seen on the extreme end opposite to the base of prism.
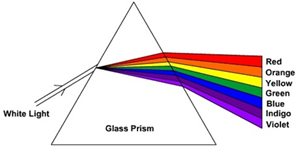
Thus, the colour of the extreme end opposite to the base of the prism is red.
Solution A.6
(d) all have the same speed
In a vacuum, all colours of light travel at the same speed. The colour we perceive is due to the wavelength of light, not its speed.
This speed is a fundamental constant of the universe and is denoted by the symbol "c" which is equal to approximately 3 x 108 meters per second.
Solution A.7
(b) Q
The critical angle of glass-air interface for yellow colour is 45°. Thus, the emergent ray for the yellow colour is along AC which is represented by ray Q.
Solution A.8
(c) 400 nm
We know,
V = nλ
V= 3 x 108 m/s
n = 7.5 x 1014 Hz
λ = (3 x 108 m/s) / (7.5 x 1014 Hz) × (1 x 109 nm / 1 m)
λ ≈ 4.00 x 10-7 meters ≈ 400 nanometres
Therefore, the wavelength of the violet light is approximately 400 nanometres.
Solution A.9
(a) δI >δG >δY >δR
The angle of deviation increases as the wavelength of light decreases. Here's the order of wavelengths for the colours:
· Red (longest wavelength)
· Yellow
· Green
· Indigo (shortest wavelength)
Therefore, the angle of deviation will be highest for Indigo and lowest for Red.
Following this order, the correct option is: δI >δG >δY >δR
Solution B.1
Speed of light increases with increase in the wavelength.
Solution B.2
Red colour travels fastest and Blue colour travels slowest in glass.
Solution B.3
Colour of light is related to its wavelength.
Solution B.4
(i) ![]()
(ii) 400 nm to 800 nm
Solution B.5
Green, Yellow orange and red have wavelength longer than blue light.
Solution B.6
A glass prism deviates the violet light most and the red light least.
Solution C.1
The deviation produced by the prism depends on the following four factors:
(a)The angle of incidence - As the angle of incidence increases, first the angle of deviation decreases and reaches to a minimum value for a certain angle of incidence. By further increasing the angle of incidence, the angle of deviation is found to increase.
(b)The material of prism (i.e., on refractive index) - For a given angle of incidence, the prism with a higher refractive index produces a greater deviation than the prism which has a lower refractive index.
(c)Angle of prism- Angle of deviation increases with the increase in the angle of prism.
(d)The colour or wavelength of light used- Angle of deviation increases with the decrease in wavelength of light.
Solution C.2
The deviation caused by a prism increases with the decrease in the wavelength of light incident on it.
Solution C.3
(a)
(i)For blue light, approximate wavelength=4800![]()
(ii)For red light, approximate wavelength=8000![]()
(b)
The colour of light with the shortest wavelength has the highest frequency.
When we compare the wavelengths of violet (4000 Å) and red (8000 Å), we notice that the wavelength of violet is shorter than that of red.
As a result, violet light of 4000 Å has a higher frequency.
Solution C.4
Seven prominent colours of the white light spectrum in order of their increasing frequencies:
Red, Orange, Yellow, Green, Blue, Indigo, Violet
Solution C.5
The seven colours in the order of increasing frequencies are red, orange, yellow, green, blue, indigo and violet.
Solution C.6
(a)In vacuum, both have the same speeds.
(b)In glass, red light has a greater speed.
Solution C.7
The phenomenon of splitting of white light by a prism into its constituent colours is known as dispersion of light.
Solution C.8
The colour band obtained on a screen on passing white light through a prism is called the spectrum.
Solution C.9
(a)Violet, Indigo, Blue, Green, Yellow, Orange, Red.
(b)No, different colours have different widths in the spectrum.
(c)(i) Violet colour is deviated the most. (ii) Red colour is deviated the least.
Solution D.1
When white light is incident on the first surface of a prism and enters in glass, light of different colours due to different speeds in glass, is refracted or deviated through different angles. Thus the dispersion of white light into its constituent colours takes place at the first surface of prism. Thus the cause of dispersion is the change in speed of light with wavelength or frequency.
Solution D.2
When white light is incident on the first surface of a prism and enters in glass, light of different colours due to different speeds in glass, is refracted or deviated through different angles. Thus the dispersion of white light into its constituent colours takes place at the first surface of prism.
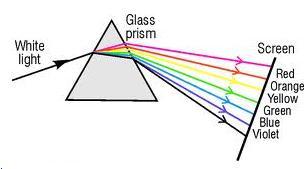
On the second surface, only refraction takes place and different colours are deviated through different angles. As a result, the colours get further separated on refraction at the second surface (violet being deviated the most and red the least).
Solution D.3
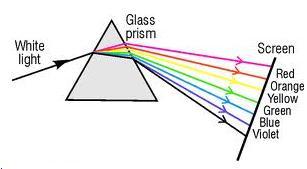
Solution D.4
(a)Constituent colours of white light are seen on the screen after dispersion through the prism.
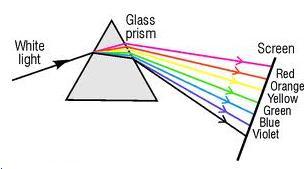
(b)When a slit is introduced in between the prism and screen to pass only the light of green colour, only green light is observed on the screen.
(c)From the observation, we conclude that prism itself produces no colour.
Solution D.5
a. If a monochromatic beam of light undergoes minimum deviation through an equi-angular prism, then the beam passes parallel to the base of prism.
b. White light splits into its constituent colours i.e., spectrum is formed.
c. We conclude that white light is polychromatic.
Solution E.1

Solution E.2
Speed of light, c= 3x 108m/s
Frequency range=3.75 x 1014Hz to 7.5 x 1014Hz.
Speed of light = frequency x wavelength
For frequency=3.75 x 1014Hz
![]()
For frequency =7.5 x 1014Hz
![]()
![]()
Spectrum Exercise Ex. 6B
Solution A.1
(d) frequency
The frequency of an electromagnetic wave is determined by the source and remains unchanged.
Solution A.2
(d) Gamma wave and infrared wave respectively
This wavelength of wave A is smaller than 0.1 A°, which is the classification for gamma rays.
This wavelength of wave B falls into the infrared range, which is typically from 700 nm (7000 A°) to 1 mm (106 A°).
Solution A.3
(c) gamma rays, X-rays, infrared rays, microwaves
The correct arrangement of the following radiations in increasing order of their wavelengths is gamma rays, X-rays, infrared rays, and microwaves.
Solution A.4
(d) Carbon arc-lamp
Solution  A.5
(b) quartz
Quartz is a type of transparent crystal that transmits ultraviolet radiation effectively. This makes it a suitable material for a prism used to obtain the ultraviolet spectrum.
Solution A.6
(a) Infrared radiation
Hint: Infrared radiations produce strong heating effect.
Solution A.7
(c) rock salt
Rock salt is a good material for an infrared prism because it transmits a wide range of infrared wavelengths with minimal absorption. This allows you to observe the full spectrum effectively.
Solution A.8
(d) Gamma rays
Gamma rays have the shortest wavelengths and the most energy of any electromagnetic wave.
Solution A.9
(a) infrared waves
Cameras can be modified to be sensitive to infrared light, which is invisible to the human eye. This allows photographers to capture images in low-light conditions where visible light is less.
Solution A.10
(a) microwaves
Microwaves are suitable for satellite communication. They can penetrate the Earth's atmosphere easily and can be focused into narrow beams for efficient transmission between the satellites and ground stations. Microwaves have higher frequencies which allow for larger data transmission capacities.
Solution A.11
(a) A-(i), B-(ii), C-(iii), D-(iv)
Solution B.1
(a) Gamma rays, X-rays, infrared rays, micro waves, radio waves.
(b) Microwave is used for satellite communication.
Solution B.2
(a) Gamma ray.
(b) Gamma rays have strong penetrating power.
Solution B.3
(a) X-rays are used in the study of crystals.
(b) It is also used to detect fracture in bones.
Solution B.4
The electromagnetic waves beyond the red end of the spectrum are known as infrared radiations.
Range: 8000 Å to 107Å (or 800 nm to 1 mm)
Solution B.5
4000![]() to 8000
to 8000![]() .
.
Solution B.6
(i)Infrared
(ii)Ultraviolet
Solution B.7
(a) infrared radiation
(b) ultra violet radiation
Solution B.8
(i)Infrared radiations are longer than 8 x 10-7m.
(ii) ultraviolet radiations are shorter than 4 x 10-7 m.
Solution B.9
(a) Ultraviolet light is obtained by passing radiations through a quartz prism.
(b) Infrared radiations is obtained by passing radiations through a rock salt prism.
Solution B.10
Water vapour, carbon dioxide, methane and ozone are the major greenhouse gases present in the Earth's atmosphere.
These greenhouse gases absorb the infrared radiations in the Earth's atmosphere.
Solution C.1
(a) Five radiations , in the order of their increasing frequencies are:
Infrared waves, Visible light, Ultraviolet, X-rays and Gamma rays.
(b) Gamma rays have the highest penetrating power.
Solution C.2
(a) The wavelength of the wave is 50 Å. Therefore, it is a X-ray.
(b) The speed of the wave will be 3 × 108 m/s in vacuum.
(c) X-rays are used to study atomic arrangement in crystals as well as complex molecules.
Solution C.3
The three radiations beyond the violet end of the visible spectrum are:
1) Ultraviolet radiations - 100 Å to 4000 Å
2) X-rays - 0.1 Å to 100 Å
3) Gamma rays - <0.1 Å
Solution C.4
The part of spectrum beyond the red and the violet ends is called the invisible spectrum as our eyes do not respond to the spectrum beyond the red and the violet extremes.
Solution C.5
(i)Ultraviolet rays-wavelength range 100 ![]() to 4000
to 4000 ![]()
(ii)Visible light-wavelength range 4000 ![]() to 8000
to 8000 ![]()
(iii)Infrared radiations-wavelength range 8000 ![]() to 107
to 107 ![]()
Solution C.6
1) Ultraviolet radiations - 100 Å to 4000 Å
Use: For detecting purity of gems, eggs, ghee, etc.
2) X-rays - 0.1 Å to 100 Å
Use: For detecting fracture in bones, teeth, etc.
Solution C.7
(i)Microwaves are used for satellite communication.
(ii)Ultraviolet radiations are used for detecting the purity of gems, eggs, ghee etc.
(iii)Infrared radiations are used in remote control of television and other gadgets.
(iv)Gamma rays are used in medical science to kill cancer cells.
Solution C.8
Lowest wavelength= gamma rays
Waves used for taking photographs= infrared rays
waves produced by the changes in the nucleus of an atom= gamma rays
waves having wavelength 0.1nm= X-rays
Solution C.9
(a)A- Gamma rays, B-infrared radiations
(b)Ratio of speeds of these waves in vacuum is 1:1 as all electromagnetic waves travel with the speed of light in vacuum.
Solution C.10
All heated bodies such as a heated iron ball, flame, fire etc., are the sources of infrared radiations.
The electric arc and sparks give ultraviolet radiations.
Solution C.11
(a)Ultraviolet radiations travel in a straight line with a speed of 3 x 108 m in air (or vacuum).
(b)They obey the laws of reflection and refraction.
(c)They affect the photographic plate.
Solution C.12
(a)Ultraviolet radiations produce fluorescence on striking a zinc sulphide screen.
(b)They cause health hazards like cancer on the body.
Solution C.13
(a)Infrared radiations travel in straight line as light does, with a speed equal to 3 x 108m/s in vacuum.
(b)They obey the laws of reflection and refraction.
(c)They do not cause fluorescence on zinc sulphide screen.
Solution C.14
They do not affect the ordinary photographic film.
Solution C.15
Ultraviolet radiation: It causes health hazards like skin cancer.
Infrared radiation: It causes skin burns.
Solution C.16
(i)Infrared radiations are used in photography in fog because they are not much scattered by the atmosphere, so they can penetrate appreciably through it.
(ii)Infrared radiations are used as signals during the war as they are not visible and they are not absorbed much in the medium.
(iii)Infrared lamps are used in dark rooms for developing photographs since they do not affect the photographic film chemically, but they provide some visibility.
(iv)Infrared spectrum can be obtained only with the help of a rock-salt prism since the rock-salt prism does not absorb infrared radiations whereas a glass prism absorbs them.
(v)A quartz prism is used to obtain the spectrum of the ultraviolet radiations as they are not absorbed by quartz, whereas ordinary glass absorbs the ultraviolet light.
(vi)Ultraviolet bulbs have a quartz envelope instead of glass as they are not absorbed by quartz, whereas ordinary glad absorbs the ultraviolet light.
Solution D.1
Infrared radiations are the electromagnetic waves of wavelength in the range of 8000![]() to 107
to 107![]() .
.
Detection: If a thermometer with a blackened bulb is moved from the violet end towards the red end, it is observed that there is a slow rise in temperature, but when it is moved beyond the red region, a rapid rise in temperature is noticed. It means that the portion of spectrum beyond the red end has certain radiations which produce a strong heating effect, but they are not visible. These radiations are called the infrared radiations.
Use: The infrared radiations are used for therapeutic purposes by doctors.
Solution D.2
The electromagnetic radiations of wavelength from 100![]() to 4000
to 4000![]() are called the ultraviolet radiations.
are called the ultraviolet radiations.
Detection: If the different radiations from the red part of the spectrum to the violet end and beyond it, are made incident on the silver-chloride solution, it is observed that from the red to the violet end, the solution remains unaffected. However just beyond the violet end, it first turns violet and finally it becomes dark brown. Thus there exist certain radiations beyond the violet end of the spectrum, which are chemically more active than visible light, called ultraviolet radiations.
Use: Ultraviolet radiations are used for sterilizing purposes.
Solution E.1
(a)Frequency =500MHz =500 x 106Hz
Wavelength= 60 cm=0.6 m
Velocity of wave= frequency x wavelength
=500x 106 x 0.6=3 x 108m/s
(b)Electromagnetic wave is travelling through air.
Solution E.2
Wavelength = 0.01![]() = 0.01 x 10-10 m
= 0.01 x 10-10 m
Speed of X-rays =3 x 108m/s
Speed of light = frequency x wavelength
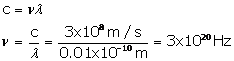
Spectrum Exercise Ex. 6C
Solution A.1
(a) smaller than
When light interacts with particles, it can be absorbed, transmitted or scattered. Scattering of light occurs when a beam of light interacts with tiny particles that are approximately smaller than the wavelength of the light. These particles cause the light to be redirected at different angles, enabling us to see objects around us.
Solution A.2
(c) I ∝ 1/ λ4
The intensity of scattered light is related to its wavelength as:
I ∝ 1/ λ4
According to Rayleigh scattering, the intensity of scattered light (I) is inversely proportional to the fourth power of the wavelength (λ).
Solution A.3
(d) Blue colour
Hint: When light of certain frequency falls on that atom or molecule, this atom or molecule responds to the light, whenever the size of the atom or molecule comparable to the wavelength of light.
The sizes of nitrogen and oxygen molecules in atmosphere are comparable to the wavelength of blue light. These molecules act as scattering centers for scattering of blue light. This is also the reason that we see the sky as blue.
Solution A.4
(c) 16
Violet light is scattered nearly 16 times more than the red light.
Solution A.5
(c) Blue
To an astronaut in a space-ship, the earth appears blue due to the large scattering of blue colour of the sunlight by the atmosphere.
Solution A.6
(d) scattering
The red colour of the sun at sunrise and sunset is due to the scattering of light. So, the answer is option (d).
Solution A.7
(d) black
There is no atmosphere on the moon. As there are no air molecules to scatter sunlight, there is no scattering of colours. Light travels in a straight line and reaches the surface of the moon. Since there's no scattered light to fill the sky, the sky on the moon appears dark or black.
Solution A.8
(b) longest, scattered
Danger signals are red because red light has the longest wavelength than the rest of visible colours. Due to its longer wavelength, red light is scattered the least by air molecules and particles in the atmosphere.
This property of red light makes it ideal for danger signals because it can travel further and be seen more clearly, especially during low visibility conditions like fog or smoke.
Note: In the textbook, the correct option given is (c) but option (b) should be the appropriate choice.
Solution B.1
Violet colour is scattered the most and red the least as the intensity of scattered light is found to be inversely proportional to the fourth power of wavelength of light.
Solution B.2
(a) Yellow light has the highest wavelength out of the three radiations. Hence, it gets scattered the least.
(b) Blue light has the lowest wavelength out of the three radiations. Hence, it gets scattered the most.
Solution C.1
When white light from sun enters the earth's atmosphere, the light gets scattered i.e., the light spreads in all directions by the dust particles, free water molecules and the molecules of the gases present in the atmosphere. This phenomenon is called scattering of light.
Solution C.2
The intensity of scattered light is found to be inversely proportional to the fourth power of wavelength of light. This relation holds when the size of air molecules is much smaller than the wavelength of the light incident.
Solution C.3
The light having the largest wavelength is scattered the least. Hence, red coloured light is scattered the least.
Solution C.4
Since the wavelength of red light is the longest in the visible light, the light of red colour is scattered the least by the air molecules of the atmosphere and therefore the light of red colour can penetrate to a longer distance. Thus red light can be seen from the farthest distance as compared to other colours of same intensity. Hence it is used for danger signal so that the signal may be visible from the far distance.
Solution C.5
On the moon, since there is no atmosphere, therefore there is no scattering of sun light incident on the moon surface. Hence to an observer on the surface of moon (space), no light reaches the eye of the observer except the light directly from the sun. Thus the sky will have no colour and will appear black to an observer on the moon surface.
Solution C.6
Scattering property of light is responsible for the blue colour of the sky as the blue colour is scattered the most due to its short wavelength.
Solution C.7
As the light travels through the atmosphere, it gets scattered in different directions by the air molecules present in its path. The blue light due to its short wavelength is scattered more as compared to the red light of long wavelength. Thus the light reaching our eye directly from sun is rich in red colour, while the light reaching our eye from all other directions is the scattered blue light. Therefore, the sky in direction other than in the direction of sun is seen blue.
Solution C.8
At the time of sunrise and sunset, the light from sun has to travel the longest distance of atmosphere to reach the observer. The light travelling from the sun loses blue light of short wavelength due to scattering, while the red light of long wavelength is scattered a little, so is not lost much. Thus blue light is almost absent in sunlight reaching the observer, while it is rich in red colour.
Solution C.9
At noon, the sun is above our head, so we get light rays directly from the sun without much scattering of any particular colour. Further, light has to travel less depth of atmosphere; hence the sky is seen white.
Solution C.10
The clouds are nearer the earth surface and they contain dust particles and aggregates of water molecules of sizes bigger than the wavelength of visible light. Therefore, the dust particles and water molecules present in clouds scatter all colours of incident white light from sun to the same extent and hence when the scattered light reaches our eye, the clouds are seen white.
Solution C.11
The smoke from the fire looks white because the size of the particle is bigger than the wavelength of light and hence it scatters light of all wavelength which makes it look white.

