Class 10 SELINA Solutions Physics Chapter 12 - Radioactivity
Radioactivity Exercise Ex. 12A
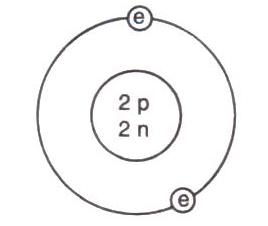
Solution A.1
(b) 2n2
The maximum number of electrons in a shell can be 2n2, where n is the number of shell.
Solution A.2
(d) both (a) and (c)
In an atom, the number of electrons equals the number of protons.
Also, the atom's atomic number equals the number of protons in its nucleus.
Solution A.3
(d) the number of neutron + number of protons
Protons and neutrons are known as nucleons since they are inside the atomic nucleus.
Thus, the total number of nucleons is also known as the atomic mass number, which is the sum of the number of protons and electrons.
Solution A.4
(b) Number of protons = A-Z
Number of electrons = Z
This statement is correct. The atomic number (Z) represents the number of protons in an atom, which is also equal to the number of electrons in a neutral atom.
Thus, (a) is correct.
(c) Number of neutrons = A - Z
This statement is correct. The mass number (A) represents an atom's total number of protons and neutrons. Since the atomic number (Z) represents the number of protons, subtracting Z from A gives the number of neutrons.
Thus, option (c) and (d) are also correct.
The only incorrect option will be option (b) because the number of protons (which is equal to the atomic number, Z) represents the number of positively charged particles in an atom. The correct relation is that the number of protons is equal to Z, not A - Z.
Solution A.5
(c) 8
Given that,
Mass number, A = 15
Atomic number, Z = 7
Thus,
Number of neutrons = A – Z = 15-7
= 8 neutrons
Thus, nucleus P will have 8 neutrons and 7 protons.
Solution A.6
(a) Isotopes
The atoms of the same element with the same atomic number Z but differ in their mass number A are called isotopes.
Example: Hydrogen (H) - 3 known isotopes (protium, deuterium, tritium)
Solution A.7
(d) Tin
Among the following tin has the largest number (=10) of isotopes.
Carbon and hydrogen have three, whereas chlorine has two.
Solution A.8
(d) Isotones
Solution A.9
(d) All of the above
All three elements listed are radioactive, i.e., their nuclei are unstable and they release energy and particles through radioactive decay.
Solution A.10
(b) α radiation
Among the following, alpha radiation will be the heaviest.
Solution A.11
(c) carbon
To study the age of excavated materials of archaeological significance, we study the rate of decay of an isotope of carbon-14 and the process is known as carbon dating.
Solution A.12
(b) one place higher
Beta particles are high-energy electrons emitted from the nucleus during radioactive decay. When a beta particle is emitted, a neutron in the nucleus is converted into a proton and an electron. This increases the atomic number of the nucleus by one, moving it one place to the right in the periodic table.
Solution A.13
(c) equal to the speed of light, i.e. 3 × 108 ms-l
Gamma rays are a form of electromagnetic radiation, and like all electromagnetic radiation, they travel at the speed of light in a vacuum, which is approximately 3 × 108 meters per second.
Solution A.14
(b) α radiation
α radiations consists of α particles, which are helium nuclei (2 protons and 2 neutrons). These are heavy and relatively slow-moving compared to other types of radiation. Their large mass and positive charge allow them to interact strongly with electrons in atoms they encounter. When such radiations travel through matter they collide and remove electrons and causes maximum ionization within a short distance.
Solution A.15
(c) ![]()
Due to γ emission, there is no change in mass and electric charge.
Hence, the correct representation of γ emission among the following is ![]() .
.
Solution B.1
Isobars
Solution B.2
Ionizing power of alpha radiation is maximum i.e., 10000 times of gamma radiation while beta particles have lesser ionizing power i.e., 100 times of gamma radiation and gamma radiation have least ionizing power.
Penetration power is least for alpha particle and maximum for gamma radiation.
Solution B.3
Speed of ![]() radiation is nearly 107 m/s.
radiation is nearly 107 m/s.
Speed of ![]() radiation is about 90% of the speed of light or 2.7 x 108 m/s.
radiation is about 90% of the speed of light or 2.7 x 108 m/s.
Speed of ![]() radiation is 3 x 108 m/s in vacuum.
radiation is 3 x 108 m/s in vacuum.
Solution B.4
Beta particle
Its symbolis ![]() .
.
Solution B.5
(a)Atomic number decreases by 2.
(b)Atomic number increases by 1.
(c)Atomic number does not change.
Solution B.6
(a)This is allowed.
(b)This is not allowed because mass number is not conserved.
Solution B.7
(a)The mass number (A) of an element is not changed when it emits beta and gamma radiations.
(b)The atomic number of a radioactive element is not changed when it emits gamma radiations.
(c)During the emission of a beta particle, the mass number remains same.
Solution B.8
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
(e)![]()
Solution B.9
Gamma radiations have very high penetration power and can easily pass through the human body. Therefore they are used as radioactive tracers in medical science.
Solution B.10
(a)![]() , (b)
, (b)![]() , (c)
, (c)![]()
The reason is that the number of neutrons exceeds the number of protons.
Solution C.1
Three constituent of an atom are:
Electrons: mass is 9.1 X10-31 kg, charge is -1.6 X 10-19C
Neutron: mass is 1.6749 X10-27 kg, charge is zero.
Protons: mass is 1.6726 X 10-27 kg, charge is +1.6 X 10-19 C
Solution C.2
Atomic number -the number of protons in the nucleus is called atomic number.
Mass number-the total number of nucleons in the nucleus is called mass number.
Solution C.3
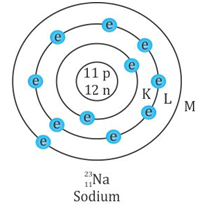
Atomic number Z = 11
Mass number A = 23
Number of neutrons A - Z = 12
Solution C.4
Isotopes: the atoms of the same element which have the same atomic number Z but differ in their mass number A are called isotopes.
Example: Hydrogen has three isotopes![]()
Solution C.5
Isobars: the atoms of different elements which have the same mass number A, but differ in their atomic number Z are called isobars.
Example: ![]()
Solution C.6
Atoms of a substance having same atomic number, but different mass numbers are called isotopes.
Example: Hydrogen has three isotopes![]()
Structure of each isotope differs by the number of neutrons in its nuclei.
Solution C.7
Radioactivity:radioactivity is a nuclear phenomenon. It is the process of spontaneous emission of ![]() radiations from the nuclei of atoms during their decay.
radiations from the nuclei of atoms during their decay.
Example: uranium, radium.
Solution C.8
There will be no change in the nature of radioactivity. This is because radioactivity is a nuclear phenomenon.
Solution C.9
This is because alpha and beta particles are charged particles, but gamma rays are neutral particles.
Solution C.10
No, it is not possible to deflect gamma radiation in a way similar to alpha and beta particles, using the electric or magnetic field because they are neutral and hence do not deflected under the action of electric or magnetic field.
Solution C.11
(a) Alpha radiations are composed two protons and two neutrons.
(b)Hydrogen has less A and Z as compared to Helium, it annot emit alpha particle.
(c) Beta particles are fast moving electrons.
Gamma radiations are photons or electromagnetic waves like X rays.
Alpha radiations have the least penetrating power.
Solution C.12
It will become singly ionized helium![]() .
.
Solution C.13
Any physical changes (such as change in pressure and temperature) or chemical changes (such as excessive heating, freezing, action of strong electric and magnetic fields, chemical treatment, oxidation etc.) do not alter the rate of decay of the radioactive substance. This clearly shows that the phenomenon of radioactivity cannot be due to the orbital electrons which could easily be affected by such changes. The radioactivity should therefore be the property of the nucleus. Thus radioactivity is a nuclear phenomenon.
Solution C.14
(a) After emitting an alpha particle the daughter element occupies two places to the left of the parent element in the periodic table.
Reason: If a parent nucleus X becomes a new daughter nucleus Y as a result of ![]() -decay, then the
-decay, then the ![]() -decay can be represented as:
-decay can be represented as:
![]()
Thus, the resulting nucleus has an atomic number equal to (Z-2). Hence, it shifts two places to the left of the parent element in the periodic table.
(b) After emitting a ![]() -particle, the daughter element occupies one place to the right of the parent element in the periodic table.
-particle, the daughter element occupies one place to the right of the parent element in the periodic table.
Reason: If a parent nucleus X becomes a new daughter nucleus Y as a result of ![]() -decay, then the
-decay, then the ![]() -decay can be represented as:
-decay can be represented as:
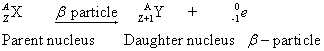
Thus, the resulting nucleus has an atomic number equal to (Z+1). Hence, it shifts one place to the right of the parent element in the periodic table.
(c) After emitting ![]() -radiation, the element occupies the same position in the periodic table.
-radiation, the element occupies the same position in the periodic table.
Reason: If a parent nucleus X becomes a new daughter nucleus Y as a result of ![]() -decay, then the
-decay, then the ![]() -decay can be represented as:
-decay can be represented as:
![]()
Thus, the resulting nucleus has atomic number equal to Z. Hence, it occupies the same position as the parent element in the periodic table.
Solution C.15
The following changes occur when an atom emits
An alpha particle: atomic number decreases by 2 and mass number decreases by 4.
Example: ![]()
A beta particle: atomic number increases by one, but mass number does not change.
Example: ![]()
Gamma particle: it does not change anything in the nucleus, the energy of the nucleus decreases.
Example: ![]()
Solution C.16
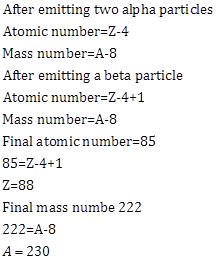
Solution C.17
Radio isotopes: The isotopes of some elements with atomic number Z
Example: carbon (Z=6, A=14).
Radio isotopes are used in medical and scientific and industrial fields. Radio isotopes such as ![]() are used as fuel for atomic energy reactors.
are used as fuel for atomic energy reactors.
Solution C.18
Because they cannot penetrate the human skin.
Solution C.19
When the number of neutrons exceeds much than the number of protons in a nuclei, it become unstable or radioactive.
Solution C.20
Many diseases such as leukemia, cancer, etc., are cured by radiation therapy. Radiations from cobalt -60 are used to treat cancer by killing the cells in the malignant tumor of the patient.
The salt of weak radioactive isotopes such as radio-sodium chloride, radio-iron and radio-iodine are used for diagnosis. Such radio isotopes are called the tracers.
Solution C.21
a < β < γ
An α-particle rapidly loses its energy as it moves through a medium and therefore its penetrating power is quite small. It can penetrate only through 3 - 8 cm in air. It can easily be stopped by a thin card sheet or a thick paper.
The penetrating power of β-particles is more than that of the α-particles. They can pass through nearly 5 m in air, through thin card sheet, and even through thin aluminium foil, but a 5 mm thick aluminium sheet can stop them.
Whereas, the penetrating power of γ-rays is high. It is about 104 times that of α-particles and 102 times that of β-particles. They can pass through 500 m in air or through 30 cm thick sheet of iron. Thick sheet of lead is required to stop them.
Solution C.22
Two main sources of nuclear radiations are:
1.Radioactive fallout from nuclear plants and other sources.
2.Disposal of nuclear waste.
These radiations are harmful because when these radiations falls on the human body, they kill the human living tissues and cause radiation burns.
Solution C.23
The following safety measures must be taken in a nuclear power plant:
1.The nuclear reactor must be shielded with lead and steel walls so as to stop radiations from escaping out to the environment during its normal operation.
2.The nuclear reactor must be housed in an airtightbuilding of strong concrete structure which can withstand earthquakes, fires and explosion.
3.There must be back up cooling system for the reactor core, so that in case of failure of one system, the other cooling system could take its place and the core is saved from overheating and melting.
Solution C.24
The radioactive material after its use is known as nuclear waste.
It must be buried in the specially constructed deep underground stores made quite far from the populated area.
Solution C.25
Three safety precautions that we would take while handling the radioactive substances are:
1. Put on special lead lined aprons and lead gloves.
2. Handle the radioactive materials with long lead tongs.
3. Keep the radioactive substances in thick lead containers with a very narrow opening, so as to stop radiations coming out from other directions.
Solution C.26
Radioactive substance should not be touched by hands because these radiations are harmful; when radiation falls on the human body, they kill the human living tissues and cause radiation burns.
Solution C.27
Background radiation: These are the radioactive radiations to which we all are exposed even in the absence of an actual visible radioactive source.
There are two sources of background radiation:
Internal source: potassium, carbon and radium are present inside our body.
External sources: cosmic rays, naturally occurring radioactive elements such as radon-222 and solar radiation.
It is not possible for us to keep ourselves away from the background radiations.
Solution D.1
The nucleus at the centre of atom, whose size is of the order of 10-15 m to 10-14 m.
The size of a nucleus is 10-5 to 10-4 times the size of an atom. It consists of protons and neutrons.
If Z is the atomic number and A is the mass number of an atom, then the atom contains Z number of electrons; Z number of protons and A - Z number of neutrons.
The atom is specified by the symbol ![]() where X is the chemical symbol for the element.
where X is the chemical symbol for the element.
Solution D.2
(a) Three types of radiations: Alpha, beta and gamma.
(b) Alpha and beta radiations
(c) Gamma radiations
(d) Gamma radiations
(e) Alpha radiations
(f) Beta radiations
Solution D.3
(a) Gamma radiations have zero mass.
(b) Gamma radiations have the lowest ionizing power.
(c) Alpha particles have lowest penetrating power.
(d) Alpha particle has positive charge equal to 3.2 x 10-19C and rest mass equal to 4 times the mass of proton i.e. 6.68 x 10-27 kg.
(e) The gas is Helium.
(f)These radiations come from nucleus of the atom.
Solution D.4
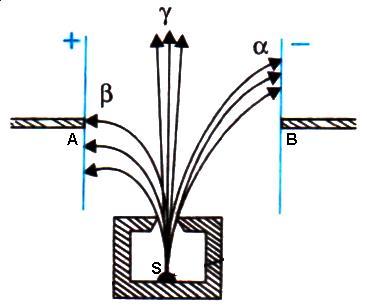
Radiations labeled A, B and C are ![]() respectively.
respectively.
Radiation labeled A is gamma radiation because they have no charge and hence under action of magnetic field they go undeflected.
Radiation B is alpha radiation because its mass is large and it would be deflected less in comparison to beta radiation. The direction of deflection is given by Fleming's left hand rule. Also directions of deflection of alpha and beta radiations are opposite as they have opposite charge.
Solution D.5
(a)
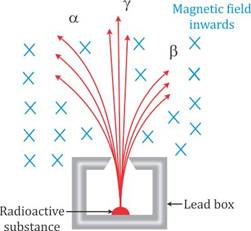
(b) Fleming's left hand rule
Solution D.6
(a)
(b)The radioactive substances are kept in thick lead containers with a very narrow opening, so as to stop radiations coming out from other directions because they may cause biological damage.
(c) Gamma radiations.
(d) Beta radiations.
(e) Alpha particle.
Solution D.7
|
Property
|
|
|
|
|
Nature
|
Stream of positively charged particles, i.e. helium nuclei.
|
Stream of negatively charged particles, i.e. energetic electrons.
|
Highly energetic electromagnetic radiation.
|
|
Charge
|
Positive charge (Two times that of a proton) = + 3.2 x 10-19 C (or +2e)
|
Negative charge = - 1.6 x 10-19 C (or -e)
|
No charge
|
|
Mass
|
Four times the mass of proton i.e., 6.68 x 10-27 kg
|
Equal to the mass of electron, i.e. 9.1 x 10-31 kg
|
No mass (Rest mass is zero)
|
|
Effect of electric field
|
Less deflected
|
More deflected than alpha particles but in direction opposite to those of
|
Unaffected
|
Solution D.8
Gamma radiation are produced when a nucleus is in a state of excitation (i.e., it has an excess of energy). This extra energy is released in the form of gamma radiation.
Gamma radiations like light are not deflected by the electric and magnetic field.
Gamma radiations have the same speed as that of light.
Solution D.9
On emitting a ![]() particle, the number of nucleons in the nucleus (i.e. protons and neutrons) remains same, but the number of neutrons is decreased by one and the number of protons is increased by one.
particle, the number of nucleons in the nucleus (i.e. protons and neutrons) remains same, but the number of neutrons is decreased by one and the number of protons is increased by one.
If a radioactive nucleus P with mass number A and atomic number Z emits a beta particle to form a daughter nucleus Q with mass number A and atomic number Z+1, then the change can be represented as follows:
![]()
(a) Atomic number 'Z' is not conserved. It is increased by 1.
(b) Mass number A is conserved.
Solution D.10
(a)The composition of B - 82 protons and 126 neutrons.
(b)The composition of C – 83 protons and 125 neutrons.
(c)The mass number of nucleus A = no. of protons +no. of neurons = 84+128=212.
(d)Their will be no change in the composition of nucleus C.
Solution D.11
(a)The alpha particle was emitted.
(b)This is because the atomic number has decreased by 2 and mass number has decreased by 4.
(c)![]()
Solution D.12
An atom is specified by the symbol ![]() where X is the chemical symbol for the element.
where X is the chemical symbol for the element.
Z is the atomic number and A is the mass number of an atom, then the atom contains Z number of electrons.
- 24 is the mass number and 11 is the atomic number.
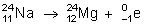
- Isobar
Solution D.13
If Z is the atomic number and A is the mass number of an atom, then the atom contains Z number of electrons; Z number of protons and A - Z number of neutrons.
The atom is specified by the symbol ![]() where X is the chemical symbol for the element.
where X is the chemical symbol for the element.
- Atomic number is 15 and mass number is 31.
- Atomic number is 15 and mass number is 32.
- Atomic number is 16 and mass number is 32.
Solution D.14
The atomic number of P decreases by 2 and mass no. decreases by 4 due to the emission of one alpha particle and then increases by 1 due to the emission of each beta particle, so the atomic number of Q formed after the emission of one alpha and two beta particles is same as that of P. Hence P and Q are the isotopes.
Radioactivity Exercise Ex. 12B
Solution A.1
(c) 931 MeV
1 amu = 931 MeV
Solution A.2
(d) 9.1×10-31
Mass of electron = 9.1×10-31
Mass of proton & Neutron ≈ 1.6725 × 10-27
Solution A.3
(d) neutron
A neutron is used in nuclear fission for bombardment.
Solution A.4
(a) E = Δmc2
According to Einstein's mass-energy equivalence:
E = mc²
Where E is energy (in MeV), m is mass (in amu), and c is the speed of light.
Solution A.5
(d) both (a) and (b)
The atomic number (Z) and mass number (A) remain conserved in each fission reaction.
Solution A.6
(d) Both (b) and (c)
In a nuclear fission reactor, the chain reaction is controlled by absorbing some of the neutrons emitted in the fission process by means of cadmium rods and slowing them down with moderators such as heavy water, graphite, etc.
Solution A.7
(c) hydrogen
The source of energy of the sun and stars is obtained from the nuclear fusion of hydrogen.
i.e., lighter nuclei, such as hydrogen, are fused into helium, producing a large amount of energy.
Solution A.8
(d) 107 K
To make the fusion possible, a high temperature of approximately 107 K and high pressure is required.
Solution B.1
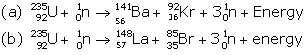
Solution B.2
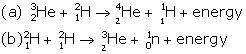
Solution B.3
- Nuclear fusion
- Nuclear fission
Solution B.4
The source of energy in the Sun and stars is the nucleus fusion of light nuclei such as hydrogen present in them in their inner part. This takes place at a very high temperature and high pressure due to which helium nucleus is formed with the release of high amount of energy.
Solution B.5
(a) Nuclear fission
(b) Nuclear fusion
Solution C.1
Energy released by combining of nuclei of an atom or by decay of an unstable radioactive nucleus during a nuclear reaction i.e., during fusion or fission is known as nuclear energy.
Solution C.2
Einstein's mass-energy equivalence relation : E = Δmc2
Where E is the energy released due to the loss in the mass Δm and c is the speed of light.
Solution C.3
- The mass of atomic particles is expressed in terms of atomic mass unit (a.m.u.). 1 a.m.u. of mass is equivalent to 931 MeV of energy.
- Mass of proton = 1.00727 a.m.u.
Mass of neutron = 1.00865 a.m.u.
Mass of electron = 0.00055 a.m.u.
Solution C.4
Nuclear fission is the process in which a heavy nucleus is splits into two light nuclei nearly of the same size by bombarding it with slow neutrons.
![]()
Solution C.5
Nearly 190 MeV of energy is released due to fission of one nucleus of ![]() . The cause of emission of this energy is the loss in mass i.e., the sum of masses of product nuclei is less than the sum of mass of the parent nucleus and neutron.
. The cause of emission of this energy is the loss in mass i.e., the sum of masses of product nuclei is less than the sum of mass of the parent nucleus and neutron.
Solution C.6
A chain reaction is a series of nuclear fissions whereby the neutrons produced in each fission cause additional fissions, releasing enormous amount of energy.
It is controlled by absorbing some of the neutrons emitted in the fission process by means of moderators like graphite, heavy water, etc. then the energy obtained in fission can be utilized for the constructive purposes
Solution C.7
(i) It is used in a nuclear bomb.
(ii) It is used in a nuclear reactor where the rate of release of energy is slow and controlled which is used to generate electric power.
Solution C.8
|
Radioactive decay |
Nuclear Fission |
|
It is a self process. |
It does not occur by itself. Neutrons are bombarded on a heavy nucleus. |
|
The nucleus emits either the a or b particles with the emission of energy in form of g rays which is not very large. |
A tremendous amount of energy is released when a heavy nucleus is bombarded with neutrons and the nucleus splits in two nearly equal fragments. |
|
The rate of radioactive decay cannot be controlled. |
The rate of nuclear fission can be controlled. |
Solution C.9
a. Nuclear fission is the process in which a heavy nucleus is splits into two light nuclei nearly of the same size by bombarding it with slow neutrons.
When uranium with Z = 92 is bombarded with neutron, it splits into two fragments namely barium (Z = 56) and krypton (Z = 36) and a large amount of energy is released which appears due to decrease in the mass.
![]()
b. Nuclear fusion is also known as thermo-nuclear reaction. This is because nuclear fusion takes place at very high temperature.
Solution C.10
When two nuclei approach each other, due to their positive charge, the electrostatic force of repulsion between them becomes too strong that they do not fuse. Thus, nuclear fusion is not possible at ordinary temperature and ordinary pressure.
Hence to make the fusion possible, a high temperature of approximately 107 K and high pressure is required. At such a high temperature, due to thermal agitations both nuclei acquire sufficient kinetic energy so as to overcome the force of repulsion between them when they approach each other, and so they get fused.
Solution C.11
![]()
- In all three deuterium nuclei fuse to form a helium nucleus with a release of 21·6 MeV energy.
- When two deuterium nuclei (
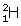 ) fuse, nucleus of helium isotope
) fuse, nucleus of helium isotope 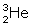 is formed and 3·3 MeV energy is released. This helium isotope again gets fused with one deuterium nucleus to form a helium nucleus
is formed and 3·3 MeV energy is released. This helium isotope again gets fused with one deuterium nucleus to form a helium nucleus 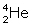 and 18·3 MeV of energy is released in this process.
and 18·3 MeV of energy is released in this process.
Solution C.12
- Both fission and fusion create release of neutrons and large amount of energy.
- Nuclear fission: A heavy nucleus splits in two nearly equal light fragments when bombarded with neutrons. It is possible at very ordinary temperature and pressureNuclear fusion: Two light nuclei combine to form a heavy nucleus at very high temperature and high pressure. Possible only at a very high temperature (≈107 K) and a very high pressure.
Solution C.13
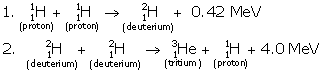
Solution D.1
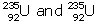
- Experimentally it is found that isotope of
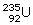 is more easily fissionable because the fission of
is more easily fissionable because the fission of 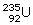 is possible by sloe neutron unlike
is possible by sloe neutron unlike 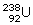 where fission is possible only by the fast neutrons.
where fission is possible only by the fast neutrons. - Slow and fast both.
Solution E.1
1 a.m.u. = 1.66 × 10-27 kg
→ 0.2 a.m.u. = 0.2 × 1.66 × 10-27 kg
Δm = 0.332 Δ 10-27 kg
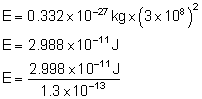
Solution E.2
Given that Δm = 0.0265 a.m.u.
1 a.m.u. liberates 931.5 MeV of energy. Thus, energy liberated equivalent to 0.0265 a.m.u. is
= 0.0265 a.m.u. × 931.5 MeV
= 24.7 meV