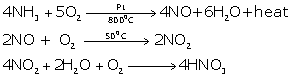Class 10 SELINA Solutions Chemistry Chapter 10 - Study of Compounds C. Nitric Acid
Study of Compounds C. Nitric Acid Exercise Intext 1
Solution 1
Cold dil. nitric acid reacts with copper to form nitric oxide.
Solution 2
(a) Aqua fortis: Nitric acid is called aqua fortis. Aqua fortis means strong water. It is so called because it reacts with nearly all metals.
(b) Aqua Regia: Conc. Nitric acid (1part by volume) when mixed with conc. Hydrochloric acid (3 parts by volume) gives a mixture called aqua regia. It means Royal water.
HNO3 +3HCl ![]() NOCl +2H2O +2[Cl]
NOCl +2H2O +2[Cl]
(c) Fixation of Nitrogen: The conversion of free atmospheric nitrogen into useful nitrogenous compounds in the soil is known as fixation of atmospheric nitrogen.
Solution 3
During lightning discharge, the nitrogen present in the atmosphere reacts with oxygen to form nitric oxide.
N2+ O2 ![]() 2NO
2NO
Nitric oxide is further oxidized to nitrogen dioxide.
2NO +O2![]() 2NO2
2NO2
The nitrogen dioxide dissolves in atmospheric moisture in the presence of oxygen of the air and forms nitric acid which is washed down by the rain and combines with the salt present on the surface of the earth.
4NO2+2H2O +O2 ![]() 4HNO3
4HNO3
Solution 4
(a) A mixture of air and dry ammonia in the ratio of 10:1 by volume
(b) Platinum gauze
(c) Oxygen
(d) ammonia : dry air :: 1 : 10
(e) Quartz is acid resistant. When packed in layers, it helps in dissolving nitrogen dioxide uniformly in water.
Solution 5
(a)Chemical equation is:
KNO3 +H2SO4 ![]() KHSO4 +HNO3
KHSO4 +HNO3
(b) Concentrated hydrochloric acid cannot replace Conc. Sulphuric acid for the preparation of nitric acid because hydrochloric acid is volatile acid and hence nitric acid vapours will carry HCl vapours.
(c) Conc. Nitric acid prepared in the laboratory is yellow in colour due to the dissolution of reddish brown coloured nitrogen dioxide gas in acid. This gas is produced due to the thermal dissociation of a portion of nitric acid.
4HNO3 ![]() 2H2O + 4NO2 + O2
2H2O + 4NO2 + O2
The yellow colour of the acid is removed:
If dry air or CO2 is bubbled through the yellow acid, the acid turns colourless because it drives out NO2 from warm acid which is further oxidized to nitric acid.
By addition of excess of water, nitrogen dioxide gas dissolves in water and thus the yellow colour of the acid is removed.
(d)The temperature of the mixture of concentrated sulphuric acid and sodium nitrate should not exceed 200oC because sodium sulphate formed at higher temperature forms a hard crust which sticks to the walls of the retort and is difficult to remove. At higher temperature nitric acid may also decompose.
NaNO3 + NaHSO4 ![]() Na2SO4 + HNO3
Na2SO4 + HNO3
Solution 6(a)
Nitric acid forms a constant boiling mixture with water containing 68% acid. This mixture boils constantly at constant boiling point without any change in its composition. At this temperature, the gas and the water vapour escape together. Hence the composition of the solution remains unchanged. So nitric acid cannot be concentrated beyond 68% by distillation of dilute solution of HNO3.
Solution 6(b)
Iron becomes inert when reacted with nitric acid due to the formation of extremely thin layer of insoluble metallic oxide which stops the reaction.
Passivity can be removed by rubbing the surface layer with the sand paper or by treating with strong reducing agent.
Solution 7
(a) When carbon and conc. Nitric acid is heated the products formed are Carbon dioxide, Nitrogen dioxide and water.
C + 4HNO3 ![]() CO2 + 2H2O +4NO2
CO2 + 2H2O +4NO2
(b) Copper when reacts with dilute HNO3 forms Copper nitrate, Nitric oxide and water.
3Cu + 8 HNO3 ![]() 3Cu(NO3) 2 +4H2O + 2NO
3Cu(NO3) 2 +4H2O + 2NO
Solution 8
(a) Reaction of nitric acid with non-metals:
C + 4HNO3 ![]() CO2 + 2H2O + 4 NO2
CO2 + 2H2O + 4 NO2
S + 6 HNO3 ![]() H2SO4 + 2H2O + 6 NO2
H2SO4 + 2H2O + 6 NO2
(b) Nitric acid showing acidic character:
K2O + 2HNO3 ![]() 2KNO3 + H2O
2KNO3 + H2O
ZnO + 2HNO3 ![]() Zn(NO3)2 + H2O
Zn(NO3)2 + H2O
(c) Nitric acid acting as oxidizing agent
P4 +20HNO3 ![]() 4H3PO4 + 4H2O + 20NO2
4H3PO4 + 4H2O + 20NO2
3Zn +8HNO3 ![]() 3Zn(NO3)2 +4H2O +2NO
3Zn(NO3)2 +4H2O +2NO
Solution 9
(a) When Sodium hydrogen carbonate is added to nitric acid sodium nitrate, carbon dioxide and water is formed.
NaHCO3 + HNO3 ![]() NaNO3 +H2O +CO2
NaNO3 +H2O +CO2
(b) When Cupric oxide reacts with dilute nitric acid, it forms Copper nitrate.
CuO +2HNO3 ![]() Cu(NO3)2 +H2O
Cu(NO3)2 +H2O
(c) Zinc reacts with nitric acid to form Zinc nitrate, nitric oxide and water.
3 Zn +8HNO3 ![]() 3Zn(NO3)2 +4H2O +2NO
3Zn(NO3)2 +4H2O +2NO
(d) 4HNO3![]() 2H2O + 4NO2 + O2
2H2O + 4NO2 + O2
Solution 10
(a) Sodium nitrate:
NaOH + HNO3 ![]() NaNO3 +H2O
NaNO3 +H2O
Sodium hydroxide reacts with nitric acid to form sodium nitrate.
(b) Copper nitrate:
CuO + 2HNO3 ![]() Cu(NO3)2 + H2O
Cu(NO3)2 + H2O
Copper oxide reacts with nitric acid to form copper nitrate.
(c) Lead nitrate:
Pb +4HNO3 ![]() Pb(NO3)2 +2H2O +2NO2
Pb(NO3)2 +2H2O +2NO2
Lead reacts with conc. nitric acid to form lead nitrate.
(d) Magnesium nitrate:
Mg +2HNO3 ![]() Mg(NO3)2 +H2
Mg(NO3)2 +H2
Magnesium with dil. nitric acid to form magnesium nitrate.
(e) Ferric nitrate:
Fe + 6HNO3 ![]() Fe(NO3)3 +3H2O + 3NO2
Fe(NO3)3 +3H2O + 3NO2
Iron reacts with conc. nitric acid to form ferric nitrate.
(f) Aqua regia:
HNO3 + 3HCl ![]() NOCl +2H2O +2[Cl]
NOCl +2H2O +2[Cl]
Nitric acid reacts with hydrochloric acid to form a mixture called aqua regia.
Solution 11
A: Copper can be converted into copper nitrate.
3Cu + 8HNO3 ![]() 3Cu(NO3)2 + 4H2O+ 2NO
3Cu(NO3)2 + 4H2O+ 2NO
B:2Cu(NO3)2 ![]() 2CuO + 4NO2 + O2
2CuO + 4NO2 + O2
C:2Cu+ O2 ![]() 2CuO
2CuO
D:By reduction
2CuO + C ![]() 2Cu + CO2
2Cu + CO2
Solution 12
(a) HNO3 is strong oxidizing agent.
(b) NaNO3 gives NaNO2 and oxygen on heating.
(c) Constant boiling nitric acid contains 68% nitric acid by weight.
(d) Nitric acid turns yellow solution when exposed to light.
(e)
Study of Compounds C. Nitric Acid Exercise Ex. 10
Solution A 1
(b) KNO3
Solution B 1
(a) Sodium nitrate
2NaNO3 ![]() 2NaNO2 +O2
2NaNO2 +O2
(b) A nitrate which on heating leaves no residue behind- Ammonium nitrate.
(c) A metal nitrate which on heating is changed into metal oxide- Calcium nitrate
(d) A metal nitrate which on heating is changed into metal- Silver nitrate
(e) A solution which absorbs nitric oxide- Freshly prepared ferrous sulphate
(f) The oxide of nitrogen which turns brown on exposure to air. - nitric oxide
By catalytic oxidation of ammonia.
4 NH3 + 5 O2 ![]() 4 NO + 6 H2O + Heat
4 NO + 6 H2O + Heat
(e) Nitrogen dioxide gas is produced when copper reacts with conc. HNO3.
Solution B 3
(i) Sulphuric acid
(ii) Nitric acid
Solution B 4
(a) Potassium nitrate
(b) Ammonium nitrate
(c) Lead nitrate
(d) Lead nitrate
Solution B 5
|
Name of Process |
Inputs |
Equations |
Output |
|
Ostwald process |
Ammonia + Air |
|
Nitric acid |
Solution C 1(a)
The glass apparatus is purposely used because nitric acid vapours are highly corrosive in nature and corrode cork, rubber etc. if used as a stopper.
Solution C 3
(a) Reddish brown gas of NO2 is observed.
(b) Yellow solid is formed which fuses with glass.
(c) When zinc nitrate crystals are strongly heated, they decompose into yellow-coloured zinc oxides and nitrogen dioxides, and oxygen gas is liberated.
(d) Reddish brown nitrogen dioxide gas is released, and the residue left behind is black copper oxide.
Solution D 1
(a) Nitrate.
(b) Sodium or potassium
(c) Lead
(d) Ammonia
(e) (1)KNO3 + H2SO4 ![]() KHSO4 + HNO3
KHSO4 + HNO3
(2) 2Pb(NO3)2 ![]() 2PbO + 4NO2 +O2
2PbO + 4NO2 +O2
(3) Cu +4HNO3 ![]() Cu(NO3)2 +H2O +2NO2
Cu(NO3)2 +H2O +2NO2
Solution D 2
(a) Three important uses of Nitric acid and the property of nitric acid involved is:
|
S.NO. |
Use |
Property |
|
1. |
To etch designs on copper and brassware. |
Nitric acid act as solvent for large number of metals. |
|
2. |
To purify gold. |
Impurities like Cu, Ag, Zn, etc. dissolve in nitric acid. |
|
3. |
Preparation of aqua regia. |
Dissolves noble metals. |
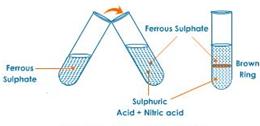
(b) Brown ring test
Procedure:
(i) Add freshly prepared saturated solution of iron (II)sulphate to the aq. solution of nitric acid.
(ii) Now add conc. Sulphuric acid carefully from the sides of the test tube, so that it should not fall drop wise in the test tube.
(iii) Cool the test tube in water.
(iv) A brown ring appears at the junction of the two liquids.
![]()
(c) A freshly prepared ferrous sulphate solution is used because on exposure to the atmosphere, it is oxidized to ferric sulphate which will not give the brown ring.
Solution D 3
(a) X is copper nitrate.
Y is nitrogen dioxide.
Z is hydrogen sulphate.
(b) ![]()
(c) ![]()
Solution D 4
(a) Dilute nitric acid is generally considered a typical acid except for its reaction with metals because it does not liberate hydrogen. It is a powerful oxidising agent, and nascent oxygen formed oxidises hydrogen in water.
(b)
i. Reaction of dilute nitric acid with copper:
3Cu + 8HNO3→ 3Cu(NO3) + 4H2O + 2NO
ii. Reaction of conc. nitric acid with copper:
Cu + 4HNO3→ Cu(NO3) + 2H2O + 2NO2
Solution D 5
(a) A (a liquid): Conc. sulphuric acid
B (a solid): Sodium nitrate
C (a liquid): Nitric acid
(b) ![]()
(c)
i. Reaction of dilute nitric acid with copper:
3Cu + 8HNO3→ 3Cu(NO3) + 4H2O + 2NO
ii. Reaction of conc. nitric acid with copper:
Cu + 4HNO3→ Cu(NO3) + 2H2O + 2NO2
Solution 2012
c. Very dilute (about 1%) acid reacts with magnesium at room temperature to give magnesium nitrate and hydrogen gas.
Solution 2013
b. First, it decomposes with slight decrepitation, and second, it is reddish brown in colour when hot. After cooling, it turns yellow and fuses in glass.
Solution 2014
a. Nitric oxide
Solution 2015 (c)
(iii) An all-glass apparatus is used in the laboratory preparation of nitric acid, because nitric acid vapour corrodes rubber and cork.
Solution A 2
(b) FeSO4
Solution A 3
(c) NO2
Solution A 4
Correct option: (b) conc. H2SO4
Solution:
In the laboratory preparation of nitric acid, nitre is heated with conc. H2SO4.
Solution A 5
Correct option: (c) Ostwald’s process
Solution:
The Ostwald process is a process used for manufacturing Nitric acid. It converts ammonia to nitric acid and involves two steps, in step 1 ammonia is oxidized to form nitric acid and nitrogen dioxide and in the second step, nitrogen dioxide is absorbed in water which in turn forms nitric acid.
Solution A 6
Correct option: (a) Pt
Solution:
The catalyst used in the manufacture of HNO3 is Platinum.
Solution A 7
Correct option: (b) NO
Solution:
Cold and dilute HNO3 oxidises metals to their nitrates and liberates nitric oxide.
The reaction is as follows:
3Cu + 8HNO3⟶ 3Cu(NO3)2 + 4H2O + 2NO
Solution A 8
Correct option: (c) NO3-
Solution:
The brown ring test is used for the detection of NO3-.
As the name suggests, it detects the presence of nitrate ions by the formation of a brown ring at the junction of two layers of the solution.
6FeSO4 + 3H2SO4 + 2HNO3⟶ 3Fe2(SO4)3 + 4H2O + 2NO
FeSO4 + NO ⟶ FeSO4.NO
Nitroso ferrous sulphate (FeSO4.NO) is a brown compound.
Solution A 9
Correct option: (c) H2SO4
Solution:
When a non-metal like sulphur reacts with concentrated nitric acid (HNO3), the sulphur oxidises to sulphuric acid (H2SO4).
The chemical reaction can be depicted as:
S (s) + 6HNO3 (aq) ⟶ H2SO4 (aq) + 2H2O (l) + 6NO2 (g)
Solution A 10
Correct option: (a) Freshly prepared FeSO4
Solution:
The solvent for NO is a freshly prepared FeSO4 solution.
Solution A 11
Correct option: (b) HNO3
Solution:
Nitric acid acts as a solvent for a large number of metals except noble metals.
Solution A 12
Correct option: (d) decomposed in the presence of light
Solution:
Nitric acid decomposes on exposure to sunlight even at room temperature turning the colour yellow due to the formation of nitrogen dioxide which dissolves in the nitric acid giving a yellow colour.
![]()
Solution A 13
Correct option: (b) NH4NO3
Solution:
Ammonium nitrate decomposes explosively on heating leaving behind no residue and produces nitrous oxide (laughing gas) and water.
![]()
Solution B 2
(a) When sulphur is treated with conc. nitric acid, it produces nitrogen dioxide gas.
(b) When a few crystals of KNO3 are heated in a hard glass test tube, it decomposes to form KNO2, and O2 gas is librated.
Solution C 1(b)
Pure nitric acid is unstable to heat or sunlight. In the presence of sunlight, it decomposes even at room temperature.
![]()
Nitric acid stored in a bottle turns yellow. This colour is due to dissolved NO2 in HNO3. To avoid decomposition, nitric acid is normally stored in coloured bottles.
Solution C 1(c)
Iron is rendered passive with fuming HNO3. This is due to the formation of insoluble metallic oxide which stops the reaction.
Solution C 1(d)
Dilute nitric acid is generally considered a typical acid but not in its reaction with metals, because the action of nitric acid on metals depends on the temperature and concentration of nitric acid. These conditions are not required in case of hydrochloric acid or sulphuric acid.
Solution C 1(e)
Although pure concentrated nitric acid is colourless, it appears yellow when left standing in a glass bottle due to the dissolution of reddish brown nitrogen dioxide gas in the acid. Nitrogen dioxide is produced because of the thermal decomposition of a portion of nitric acid.
4HNO3 → 2H2O + 4NO2 + O2
Solution C 2
The dilute acid is nitric acid.
Reaction of dilute nitric acid with copper:
3Cu + 8HNO3 →3Cu(NO3) + 4H2O + 2NO
Solution C 4
Its oxidising property allows it to react with copper.
Solution C 5
(a) ![]()
(b) ![]()
(c) ![]()
(d) Reaction of dilute nitric acid with copper:
3Cu + 8HNO3→ 3Cu(NO3) + 4H2O + 2NO Reaction of conc. nitric acid with copper:
Cu + 4HNO3→ Cu(NO3) + 2H2O + 2NO2