Class 10 SELINA Solutions Chemistry Chapter 9 - Study of Compounds B. Ammonia
Study of Compounds B. Ammonia Exercise Intext 1
Solution 1(a)
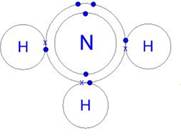
Covalent bonding is present in ammonia.
Solution 1(b)
Formula of liquid ammonia is: NH3.
Liquid ammonia is liquefied ammonia and is basic in nature. It dissolves in water to give ammonium hydroxide which ionizes to give hydroxyl ions.
NH3 + H2O ![]() NH4OH
NH4OH
NH4OH ![]() NH4++ OH-
NH4++ OH-
Therefore it turns red litmus blue and phenolphthalein solution pink.
Solution 2
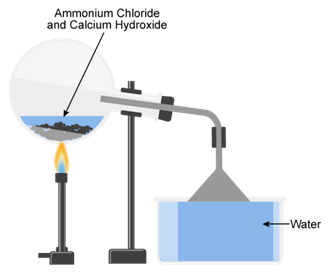
(a) Lab preparation of ammonia:
2NH4Cl+Ca(OH)2 ![]() CaCl2 +2H2O +2NH3
CaCl2 +2H2O +2NH3
(b) The ammonia gas is dried by passing through a drying tower containing lumps of quicklime (CaO).
(c) Ammonia is highly soluble in water and therefore it cannot be collected over water.
Solution 3
(a) An aqueous solution of ammonia is prepared by dissolving ammonia in water. The rate of dissolution of ammonia to water is very high.
Diagram:
(b)The drying agent used is CaO in case of ammonia.
Other drying agents like P2O5 and CaCl2 are not used. As ammonia being basic reacts with them.
6NH3 + P2O5 +3H2O ![]() 2(NH4)3PO4
2(NH4)3PO4
CaCl2 +4NH3 ![]() CaCl2.4NH3
CaCl2.4NH3
Solution 4
The substance A is Ammonium chloride and 'B' is Ammonia.
Reaction:
2NH4Cl + Ca(OH)2 ![]() CaCl2 + 2H2O + 2NH3
CaCl2 + 2H2O + 2NH3
Solution 5
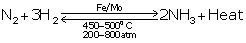
(a) Conditions for reactants to combine :
Optimum temperature is 450o-500oC
Above 200 atm pressure
Finely divided iron as catalyst
Traces of molybdenum or Al2O3 as promoters.
Reaction:N2 +3H2 ![]() 2NH3 + heat
2NH3 + heat
(b) Dry nitrogen and dry hydrogen in the ratio of 1:3 by volume is made to combine.
(c) Source of Hydrogen: Hydrogen is generally obtained from water gas by Bosch process.
(CO + H2) + H2O ![]() CO2 +2H2
CO2 +2H2
Source of Nitrogen: It is obtained from fractional distillation of liquid air.
(d) High pressure favours the forward reaction i.e. formation of ammonia.
(e)Two possible ways by which NH3 produced is removed from unreacted N2 and H2 by:
(i)Liquefaction: NH3 is easily liquefiable.
(ii)Absorbing in water: As ammonia is highly soluble in water.
(f)
(i)Finely divided iron increases the rate of reaction.
(ii)Molybdenum acts as a promoter to increase the efficiency of the catalyst.
(g) 15%
(h) The unchanged nitrogen and hydrogen are recirculated through the plant to get more ammonia. By recirculating in this way, an eventual yield of 98% can be achieved.
Solution 6
(a) Ammonium compounds being highly soluble in water do not occur as minerals.
(b) Ammonium nitrate is not used in the preparation of ammonia as it is explosive in nature and it decomposes forming nitrous oxide and water vapours.
(c) Conc. H2SO4 is not used to dry ammonia, as ammonia being basic reacts with them.
2NH3 + H2SO4 ![]() (NH4)2SO4
(NH4)2SO4
(d) (i) In order to better mixing of ammonium chloride, calcium hydroxide used in excess.
(ii) The flask is fitted in slatting position because of the water formed in the reaction does not trickle back into the heated flask.
Solution 7
(a) 450 - 500°C
(b) 200 -800 atm
(c) Finely divided iron (Fe)
Solution 8
a. Neutralisation
b. Thermal decomposition
c. Ammonia
Solution 9
An element has 2 electrons in its N shell = Ca (calcium)
It reacts with a non-metal of atomic number 7 = N (nitrogen)
3Ca (s) + N2 (g) → Ca3N2
The compound formed is calcium nitride (Ca3N2) which reacts with warm water and produces the basic gas ammonia (NH3).
Ca3N2 + 6H2O → 3Ca(OH)2 + 2NH3
Solution 10
(a) The formula of the compound is Mg3N2.
(b) Balanced equation :
Mg3N2
+ 6 H2O ![]() 3 Mg(OH)2 + 2 NH3
3 Mg(OH)2 + 2 NH3
(c) Ammonia is a reducing agent and reduces less active metal oxide to its respective metal.
Solution 11
a. A reddish brown precipitate is obtained when ammonium hydroxide is added to ferrous chloride.
b. Aqueous ammonia is a solution of NH3.
c. Finely divided iron is used in Haber process.
d. Quicklime (CaO) is a drying agent for NH3.
e. Ammonium salts, on thermal decomposition, give ammonia and hydrogen chloride.
Solution 12
C.: Magnesium nitride
Study of Compounds B. Ammonia Exercise Ex. 9
Solution A 1
Correct option: (d) Iron
Solution:
The catalyst used in Haber’s process of preparation of ammonia is finely divided iron.
Solution A 2
Correct option: (b) Ammonium nitrite
Solution:
Solution:
(a) Ammonium nitrate
![]()
(b) Ammonium nitrite
![]()
(c) Magnesium nitride (Rare condition)
![]()
(d) Ammonium chloride
![]()
Solution A 3
(c) Mg(NO3)2
Solution A 4
Correct option: (b) NH3
Solution:
Ammonia is not collected over water since it is highly soluble in water. Ammonia gas is lighter than air and hence collected by the downward displacement of air.
Solution A 5
Correct option: (c) 1:3
Solution:
In Haber’s Process, nitrogen gas and hydrogen gas are taken in a ratio of 1:3 by volume.
The reaction of nitrogen with hydrogen in Haber’s process is as follows.
N2(g) + 3H2(g) ⟶ 2NH3(g)
Solution A 6
Correct option: (b) NCl3
Solution:
When ammonia gas reacts with excess chlorine (Cl2) gas, it produces nitrogen trichloride (NCl3) liquid and hydrogen chloride (HCl) gas.
The chemical reaction is as follows:
NH3(g) + 3Cl2(g) ⟶ NCl3(l) + 3HCl (g)
Solution A 7
Correct option: (c) Ostwald process
Solution:
Ammonia is used in Ostwald’s process for the production of nitric acid.
Solution B 1
(a) Ammonia
(b) Hydrogen chloride and chlorine gas.
(c) (i) Ammonium chloride
(i) Ammonium nitrate
(ii) Ammonium carbonate
(d) Acidic gas: HCl
Basic gas: Ammonia
Neutral gas: NH4Cl
(e) Silver chloride
(f) Nitrogen
(g) Magnesium nitride
(h) Lead oxide
(i) Ammonium chloride
(j) Ammonia
(k) Nitrogen
(l) Ammonium chloride.
(m) Ammonia
Solution B 2
(a) Ammonia gas is collected by downward displacement of air.
(b) Ammonia reacts with excess chlorine to form nitrogen trichloride.
Solution B 3
i. Ammonia
ii. Alkaline
iii. Ammonium
iv. Hydroxyl
v. Dirty green
Solution B 4
(a) Ammonia
(b) Nitrogen
Solution B 5
(a) Explosive: ammonium nitrate
(b) Medicine: ammonium carbonate
(c) Fertilizers: ammonium sulphate
(d) Laboratory reagent: ammonia solution
Solution B 6
Ammonia (NH3)
Solution C 1
(a) Ammonia is basic in nature.
(b) Copper oxide because CuO is less reactive can be reduced by C, CO or by hydrogen whereas Al2O3, Na2O, MgO are reduced by electrolysis.
Solution C 2
(a) The gas is ammonia.
(b) The formula is NH3.
(c) Uses of ammonia:
It is used in the industrial preparation of nitric acid by Ostwald process.
It is used in the manufacture of fertilizers such as ammonium sulphate, ammonium nitrate, ammonium phosphate.
It is used in the manufacture sodium carbonate by Solvay process.
NaCl + NH3 + CO2 + H2O ![]() NaHCO3 +NH4Cl
NaHCO3 +NH4Cl
Solution C 3
(a) AlN + 3H2O ![]() Al(OH)3 +NH3
Al(OH)3 +NH3
(b) 2NH3 + 3PbO ![]() 3Pb + 3H2O + N2
3Pb + 3H2O + N2
(c) 8NH3 +3Cl2 ![]() N2 + 6NH4Cl
N2 + 6NH4Cl
(d) 2NH3 + CO2 ![]() NH2CONH2 + H2O
NH2CONH2 + H2O
(i) Ammonia act as reducing agent is explained by equation (c).
(ii) Urea the nitrogenous fertilizer is prepared from equation (d).
Solution C 4(a)
(i) Dirty green ppt. of Ferrous hydroxide is formed which is insoluble in excess of NH4OH.
FeSO4 + 2NH4OH ![]() [NH4]2SO4 + Fe(OH)2
[NH4]2SO4 + Fe(OH)2
(ii) Reddish brown ppt. of ferric hydroxide is formed which is insoluble in ammonium hydroxide.
FeCl3 + 3NH4OH ![]() 3NH4Cl + Fe(OH)3
3NH4Cl + Fe(OH)3
(iii) White ppt. of lead hydroxide is formed which is insoluble in NH4OH.
Pb(NO3)2 + 2NH4OH ![]() 2NH4NO3 + Pb(OH)2
2NH4NO3 + Pb(OH)2
(iv) White gelatinous ppt. of Zinc hydroxide is formed which is soluble in NH4OH.
Zn(NO3)2 + 2NH4OH ![]() 2NH4NO3 + Zn(OH)2
2NH4NO3 + Zn(OH)2
Solution C 5
CuSO4 + 2NH4OH ![]() (NH4)2SO4 +Cu(OH)2 [Pale blue]
(NH4)2SO4 +Cu(OH)2 [Pale blue]
The cation present in solution B is Copper (Cu+2).
The colour of solution B is Blue.
The pale blue precipitate of copper hydroxide dissolves in excess of ammonium hydroxide forming tetraamine copper[II] sulphate, an azure blue(deep blue) soluble complex salt.
Cu(OH)2 + (NH4)2SO4 +2NH4OH ![]() [Cu(NH3)4]SO4 + 4H2O
[Cu(NH3)4]SO4 + 4H2O
Solution C 6
(a) Ammonium chloride
NH4Cl ![]() NH3 +HCl
NH3 +HCl
(b) Ammonium nitrate
NH4NO3 ![]() N2O +2H2O
N2O +2H2O
Both are examples of Thermal dissociation.
Solution C 7
i. Ammonia and hydrogen chloride gas
ii. High solubility of gases in water
Solution C 8
i. Hydroxyl (OH-) ion other than ammonium ion
ii. Red litmus turns blue, methyl orange turns yellow and phenolphthalein turns pink.
Solution C 9
In the presence of platinum catalyst at 800°C.
Solution C 10
(a) Liquid ammonia acts as a refrigerant in ice plants. Evaporation needs heat energy, and when liquid ammonia vaporises, it absorbs large quantities of heat without changing its temperature. For these reasons, ammonia is widely used as a refrigerant.
(b) Ammonia is used for removing grease and dirt as it emulsifies or dissolves them.
(c) Ammonia is formed by bacterial decomposition of urea, so ammonia has a pungent smell.
(d) An aqueous solution of ammonia is a weak electrolyte. It dissociates partially to give hydroxyl ions, and ions conduct electricity. So an aqueous solution of ammonia conducts electricity.
Solution C 11(a)
Ammonium hydroxide is added to ferrous sulphate solution.
2NH4OH + FeSO4⟶ Fe(OH)2 ↓ + (NH4)2SO4
Solution C 11(b)
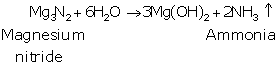
Solution C 11(c)
![]()
Solution C 11(d)
![]()
Solution C 11(e)
![]()
Solution C 11(f)
![]()
Solution C 11(g)
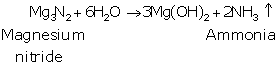
Solution C 11(h)
2NH4Cl + Ca (OH)2 → CaCl2 + 2H2O + 2NH3
Solution C 11(i)
NH3 + 3Cl2→ NCl3 + 3HCl
(Nitrogen trichloride)
Solution C 11(j)
2NH3 + H2SO4→ (NH4)2SO4
Ammonium sulphate
Solution C 11(k)
8NH3 + 3Cl2→ N2 + 6NH4Cl
Solution C 11(l)
3PbO + 2NH3→3Pb + 3H2O + N2
Solution C 12
Balanced equation:
(a) 2NH3 +3CuO ![]() 3Cu +3H2O +N2
3Cu +3H2O +N2
(b) 2NH3 +3Cl2 ![]() N2 + 6HCl
N2 + 6HCl
Solution C 13
![]()
Solution C 14
Magnesium nitride reacts with boiling water to liberate ammonia.
Solution C 15
(a) Calcium chloride and zinc chloride
Calcium chloride does not react with ammonium hydroxide.
On adding ammonium hydroxide drop by drop to a solution of zinc salt a white precipitate is formed which is soluble in excess of ammonium hydroxide.
(b) Ferric salt and ferrous salt:
When ammonium hydroxide is added drop wise to solution to be tested.
Ferrous salt gives dirty green ppt.
Ferric salt gives reddish brown ppt of their hydroxides.
(c) Zinc nitrate and lead nitrate
When ammonium hydroxide is added drop wise to solution to be tested.
Zinc nitrate gives white gelatinous ppt. of their hydroxides which is soluble on adding excess of ammonium hydroxide.
Whereas, lead nitrate gives white ppt. of their hydroxides which is insoluble on adding excess of ammonium hydroxide.
Solution C 16
(a) 8NH3 + 3Cl2 → N2 + 6NH4Cl
(b) 4NH3 + 5O2 ![]() 4NO + 6H2O + Heat
4NO + 6H2O + Heat
2NO + O2 → 2NO2
(c) NH3 + 3Cl2 → 3HCl + NCl3
(d) AlCl3 + 3NH4OH → 3NH4Cl + Al(OH)3
(e) AlN + 3H2O → Al(OH)3 + NH3
(f) 3PbO + 2 NH3 → 3Pb + 3H2O + N2
Solution C 17
i. When added in small quantity, it forms a gelatinous white ppt.
ii. When added in excess, it dissolves to form a complex salt.
Solution C 18
|
Name of the process |
Temperature |
Catalyst |
Equation for the catalysed reaction |
|
Haber's process |
450-500°C |
Finely divided iron (Fe) |
|
Solution C 19
(a) Iron(II) sulphate: Gives dirty green ppt. with ammonium hydroxide insoluble in excess.
(b) Iron(III) sulphate: Gives reddish brown ppt. with ammonium hydroxide insoluble in excess.
Solution C 20(a)
When ammonia gas is passed over heated copper (II) oxide, black copper oxide is reduced to greyish metallic copper.
Solution C 20(b)
Ammonia burns with a yellowish flame. It produces water vapour and nitrogen.
Solution C 20(c)
In small quantity: A bluish white ppt. is obtained.
In excess quantity: A deep blue solution is obtained.
Solution C 20(d)
Pungent smelling basic gas called ammonia is evolved.
Solution C 20(e)
A yellow-coloured explosive is formed.
Solution C 20(f)
A colourless pungent-smelling ammonia gas is obtained.
Solution D 1
(a)Ammonia is less dense than air. By Fountain Experiment, we demonstrate the high solubility of ammonia gas in water.
(b) The high solubility of ammonia gas in water
(c)The balanced equation for the reaction between ammonia and sulphuric acid is:
2NH3 + H2SO4 ![]() (NH4)2SO4
(NH4)2SO4
Solution D 2
Equation:
CuSO4 +2NH4OH![]() Cu(OH)2
Cu(OH)2![]() + [NH4]2SO4
+ [NH4]2SO4
pale blue
Ammonia solution in water gives a blue precipitate when it combines with a solution of copper salt.
The pale blue precipitate of copper hydroxide dissolves in excess of ammonium hydroxide forming tetraamine copper[II] sulphate, an azure blue(deep blue)soluble complex salt.
Cu(OH)2 +(NH4)2SO4 +2NH4OH ![]() [Cu(NH3)4]SO4 + 4H2O
[Cu(NH3)4]SO4 + 4H2O
Solution D 3
![]()
This reaction is reversible and exothermic. From the reaction, it is proved that ammonia contains nitrogen and hydrogen.
Also,
![]()
Ammonia burns with a yellowish flame. It produces water vapour and nitrogen. This shows that ammonia contains nitrogen and hydrogen.
Solution D 4
Three ways in which ammonia gas can be identified is:
It has a sharp characteristic odour
When a glass rod dipped in HCl is brought in contact with the gas white colour fumes of ammonium chloride are formed
It turns moist red litmus blue, moist turmeric paper brown and phenolphthalein solution pink.
Solution D 5
As the 'A' turns red litmus blue it is a base.Now the gas 'A' combines with 'B' in presence of Catalyst to give colourless gas Nitrogen monoxide. It reacts with oxygen to give brown gas which is Nitrogen dioxide.
A= NH3
B= O2
C=NO
D=NO2
Reactions:
4NH3 + 5O2 ![]() 4NO + 6H2O + Heat
4NO + 6H2O + Heat
2NO + O2 ![]() 2NO2
2NO2
NH3 in water forms NH4OH which turns red litmus blue.
Solution D 6
(a) The main refrigerants used are Freon chlorofluorocarbons (CFC). They deplete ozone layer. The chlorofluorocarbons are decomposed by ultraviolet rays to highly reactive chlorine which is produced in the atomic form.
![]()
The free radical [Cl] reacts with ozone and chlorine monoxide is formed.
![]()
This causes depletion of ozone layer and chlorine monoxide so formed reacts with atomic oxygen and produces more chlorine free radicals.
ClO + O![]() Cl + O2
Cl + O2
Again this free radical destroys ozone and the process continues thereby giving rise to ozone depletion.
(b) Liquid ammonia can be used as a refrigerant, as an alternative for chlorofluorocarbons.
(c) Advantages of ammonia as refrigerant:
(i) Ammonia is environmentally compatible. It does not deplete ozone layer and does not contribute towards global warming.
(ii) It has superior thermodynamic qualities as a result ammonia refrigeration systems use less electricity.
Ammonia has a recognizable odour and so leaks are not likely to escape.
Solution D 7
(a)It is the basic nature of ammonia molecule.
(b)Hydroxyl ion (NH3 +H2O ![]() NH4+ + OH-)
NH4+ + OH-)
(c) The red litmus paper turns blue in the solution.
Solution D 8
(a) HCl gas is more dense [V.D.=18.25,V.D. of ammonia =8.5] and it is collected by the upward displacement of air.
(b) NH3 + HCl → NH4Cl
Solution D 9
A. Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
B. NH3 + HCl → NH4Cl
Or
8NH3 + 3Cl2 → 6NH4Cl + N2
C. NH4Cl + NaOH → NaCl + NH3 + H2O
Solution D 10
(a) Haber's process
(b) 1 part of nitrogen gas and 3 parts of hydrogen gas
(c) Finely divided iron (Fe)
(d) ![]()
Solution D 11
(a) Ammonium nitrate is a highly explosive substance and cannot be heated.
(b) Quicklime/CaO
(c) By the downward displacement of air or upward delivery as it is lighter than air.
It is not collected over water because it easily dissolves in water.
Solution D 12
(a) Ammonia
(b) ![]()
(c) By downward displacement of air
(d) Quicklime/CaO
(e) Bring a moist red litmus paper to the mouth of the inverted jar if it immediately turns blue.
Or
Bring a glass rod dipped in hydrochloric acid to the mouth of the inverted jar. If it produces dense white fumes, then the jar is full of gas.
Solution A 3
Correct option: (b) Mg3N2
Solution:
Ammonia is obtained by adding water to magnesium nitride.
The chemical equation for this reaction is,
Mg3N2(s) + 6H2O(l) ⟶ 2NH3(aq) + 3Mg(OH)2(aq)