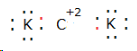Class 10 SELINA Solutions Chemistry Chapter 1 - Periodic Table, Periodic Properties and Variations of Properties
Periodic Table, Periodic Properties and Variations of Properties Exercise Intext 1
Solution 1
(i) The modern periodic law states that "The properties of elements are the periodic functions of their atomic number."
(ii) Henry Moseley put forward the modern periodic law.
(iii) Modern Periodic table has 7 periods and 18 groups.
Solution 2
The horizontal rows are known as periods and vertical columns in the periodic table are known as groups.
Solution 3
Periodicity is observed due to the similar electronic configuration.
Solution 4
(i) Though the number of shells remain the same, number of valence electrons increases by one, as we move across any given period from left to right.
(ii) While going from top to bottom in a group, the number of shells increases successively i.e. one by one but the number of valence electrons remains the same.
Solution 5
(i) Sodium and Potassium
(ii) Calcium and Magnesium
(iii) Chlorine and Bromine
(iv) Neon and Argon
Solution 6
Valency is the combining capacity of the atom of an element. It is equal to the number of electrons an atom can donate or accept or share. It is just a number and does not have a positive or negative sign.
Group 1 elements have 1 electron in their outermost orbital, while Group 7 elements have 7 electrons in their outermost orbital.
Valency depends on the number of electrons in the outermost shell (i.e. valence shell).
If the number of electrons present in the outermost shell is 1, then it can donate one electron while combining with other elements to obtain a stable electronic configuration.
If the number of electrons present in the outermost shell is 7, then its valency is again 1 (8 - 7 = 1) as it can accept 1 electron from the combining atom in order to achieve stable electronic configuration.
In a given period, the number of electrons in the valence (outermost) shell increases from left to right. But the valency increases only up to Group 14, where it becomes 4, and then it decreases, that is, it becomes 1 in Group 17.
Solution 7
(i) Elements in the same group have the same valency.
(ii) Valency depends upon the number of valence electrons in an atom.
(iii) Copper and zinc are transition elements.
(iv) Noble gases are placed at the extreme right of the periodic table.
Solution 8
(i) The properties that reappear at regular intervals, or in which there is a gradual variation at regular intervals, are called periodic properties and the phenomenon is known as the periodicity of elements.
(ii) The third-period elements, Na, Mg, Al, Si, P and Cl summarize the properties of their respective groups and are called typical elements.
(iii) Electrons revolve around the nucleus in certain definite circular paths called orbits or shells.
Solution 9
Beryllium and magnesium will show similar chemical reactions as calcium. Since these elements belong to same group 2 andalso have two electrons in their outermost shell like calcium.
Solution 10
- Metals: Lithium, Beryllium, Sodium, Magnesium, Aluminium, Potassium, Calcium
- Metalloids: Boron, Silicon
- Non-metals: Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorous, Sulphur, Chlorine, Argon
Solution 11
Solution 12
The main characteristic of the last element in each period of the periodic table is they are inert or chemically unreactive.
The general name of such elements is 'Noble gases'.
Solution 13
According to atomic structure, the number of valence electrons determines the first and the last element in a period.
Solution 14
i. The valence electrons increase from 1 to 8 in the 3rd period of the periodic table.
ii. On moving from left to right, the valency increases from 1 to 4 and then decreases from 4 to 0.
Solution 15
(i) Noble gases
(ii) Representative elements
(iii) Transition elements
(iv) Halogens
(v) Alkaline Earth metals
Solution 16
(i) 30
(ii) It belongs to group 12 and fourth period.
(iii) It is a metal.
(iv) The name assigned to this group is IIB.
(v) 2
Solution 17
(i) Electronic configuration of S: 2,8,6
(ii) 16th Group and 3rd Period.
(iii) Valency of S = 8 - 6 = 2
(iv) Sulphur is a non-metal.
(v) It is an oxidizing agent.
(vi) Formula with hydrogen = H2S
Solution 18
- Na and F
- Argon
- C, N, O and F are non-metals present in period 2 while Na, Mg and Al are metals in period 3.
- Silicon
- Argon
- Mg
Solution 19
- Group = 1
- Period = 4
- Valence electrons = 1
- Valency = 1
- Metal
Solution 20
(i) A metal of valency one = 19
(ii) A solid non-metal of period 3 = 15
(iii) A rare gas = 2
(iv) A gaseous element with valency 2 = 8
(v) An element of group 2 = 4
Periodic Table, Periodic Properties and Variations of Properties Exercise Intext 2
Solution 1
Atomic size is the distance between the centre of the nucleus of an atom and its outermost shell.
It's measured in Angstrom and Picometre.
Solution 2
(i) The atomic size of an atom increases when we go down a group from top to bottom.
(ii) It decreases as we move from left to right in a period.
Solution 3
Second Period: Fluorine < Oxygen < Nitrogen < Carbon < Boron < Beryllium < Lithium.
Third Period: Chlorine < Sulphur < Phosphorus < Silicon < Aluminum < Magnesium < Sodium.
Solution 4
(i) The size of Neon is bigger compared to fluorine because the outer shell of neon is complete(octet).As a result, the effect of nuclear pull over the valence shell electrons cannot be seen. Hence the size of Neon is greater than fluorine.
(ii) Since atomic number of magnesium is more than sodium but the numbers of shells are same, the nuclear pull is more in case of Mg atom. Hence its size is smaller than sodium.
Solution 5
(i)
a. An atom is always bigger than cation since cation is formed by the loss of electrons; hence protons are more than electrons in a cation. So the electrons are strongly attracted by the nucleus and are pulled inward.
b. An anion is bigger than an atom since it is formed by gain of electrons and so the number of electrons are more than protons. The effective positive charge in the nucleus is less, so less inward pull is experienced. Hence the size expands.
c. Fe2+ is bigger than Fe3+ since Fe2+ has more number of electrons than Fe3+ and hence the inner pull by nucleus is less strong on it as compared to the pull on Fe3+.
(ii) Fluorine
(iii) K
Solution 6
- In increasing metallic character: F < O < N < C < B < Be < Li
- In decreasing non-metallic character: Cl > S > P > Si > Al > Mg > Na
Solution 7 (i)
Across the third period left to right
Across a period, the chemical reactivity of elements first decreases and then increases.
The group 1 element can lose electrons easily as compared to group 2. Similarly, group 2 elements can also lose electrons easily but only in comparison to group 13 and not at all in comparison to group 1. As the tendency to lose electrons decreases, reactivity also decreases and thus silicon is the least reactive element in the third period. As we move from phosphorus to chlorine, the tendency to gain electrons increases, hence reactivity again increases in the third period.
Na Mg Al Si P S Cl
Most ⟶ least ⟵ Most
reactive reactive reactive
Metal Non-metal
Solution 7 (ii)
Down the group,
a. In group IA (1)
The tendency of losing electrons increases down the group. Since chemical reactivity in metals depends upon the tendency to lose electrons, thus reactivity increases on going down the group.
The most reactive metal is at the bottom of group 1.
b. In group VIIA (17)
The chemical reactivity of non-metals decreases on going down the group as it depends upon the tendency to gain electrons, which decreases down the group. The most reactive non-metal is at the top of group 17 i.e., Fluorine.
Solution 8
Given: The metal belongs to the third period; there are three shells.
The chemical formula of the compound suggests that the valency of the metal is +3.
That means the valence electrons are 3; hence, it belongs to the third group.
Thus, the element must have the electronic configuration 2, 8, 3.
That means the total number of electrons is 13.
Valency = 3, Atomic number = 13
Solution 9
(i) The element from the 17th group has 7 electrons in its outermost shell.
(ii) The name of the element is chlorine.
(iii) Chlorine belongs to the halogen family.
(iv) The element has![]() three electrons in its outermost shell which it can donate; hence, its valency is three. While the valency of chlorine is 1. Thus,
three electrons in its outermost shell which it can donate; hence, its valency is three. While the valency of chlorine is 1. Thus, ![]() which is Aluminium can donate three electrons, and chlorine can accept 1 electron to get the stable electronic configuration.
which is Aluminium can donate three electrons, and chlorine can accept 1 electron to get the stable electronic configuration.
Therefore, the formula of the compound is AlCl3.
Solution 10
(i) Yes, these elements belong to the same group but are not from the same period.
(ii) We know that m.p. decreases on going down the group. Hence, from the above table, the elements can be ordered according to their period as follows:
|
Elements |
B |
C |
A |
|
m.p. |
180.0 |
97.0 |
63.0 |
The metallic character increases as one moves down the group.
Hence, the order of the given elements with increasing metallic character is as follows:
B
Solution 11
Correct option: (ii) Potassium
Solution 12
Correct option: (iii) I-
Solution 13
(i) Barium will form ions most readily as the outermost valence electron which experiences the least force of attraction by positively charged nucleus can be given away readily to form cations.
(ii) All Group II elements have two valence electrons.
Solution 14
![]()
Protons = 19, Neutrons = 39 - 19 = 20
Electronic configuration = 1s22s22p63s23p64s1
Position in the periodic table = Group 1, Period 4
![]()
Protons = 15, Neutrons = 31 - 15 =16
Electronic configuration = 1s2 2s2 2p6 3s2 3p3
Position in the periodic table = Group 3, Period 3
Solution 15
(i) Neon (Atomic number = 10)
Electronic configuration = 1s22s22p6
(ii) Electronic configuration = 2, 8, 3
Hence, atomic number = 13
The element having atomic number 13 is Aluminium.
(iii) The element has a total of three shells; hence, the element belongs to the third period. Five valence electrons indicate that the element belongs to the fifth group (VA). Hence, the element is phosphorus.
(iv) Twice as many electrons in its second shell as in its first shell indicates electronic configuration 1s22s2.
(v) From the electronic configuration, the total number of electrons is 4. We know that,
Number of electrons = Number of protons = Atomic number
The element with atomic number 4 is beryllium.
Solution 16
(i) It belongs to group II and has 2 valence electrons, so it is a metal.
(ii) Barium is placed below calcium in the group. Since the reactivity increases below the group, barium is more reactive than calcium.
(iii) It needs to lose 2 valence electrons to complete its octet configuration, so its valency is 2.
(iv) The formula of its phosphate will be Ba3 (PO4)2.
(v) As we move from left to right in a period, the size decreases, so it will be smaller than caesium.
Solution 17
Since, the size of the atom increases down the group, the ionic radii will also increase. Hence, the order of increasing atomic numbers in the group is Z < Y < X.
Solution 18
(i) All groups do not contain both metals and non-metals. Group I and II contain only metals.
(ii) Atoms of elements in the same group have same number of valence electrons. They have same number of electrons present in their outermost shell.
(iii) The non-metallic character increases across a period with increase in atomic number. This is because across the period, the size of atom decreases and the valence shell electrons are held more tightly.
(iv) On moving from left to right in a period, the reactivity of elements first decreases and then increases, while in groups, chemical reactivity of metals increases going down the group whereas reactivity of non-metals is decreases down the group.
Solution 19
- Period 1:
Number of elements = 2
Hydrogen, helium
Period 2:
Number of elements = 8
Lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, neon
Period 3:
Number of elements = 8
Sodium, magnesium, aluminium, silicon, phosphorus, sulphur, chlorine, argon
- A common feature of the electronic configuration of the elements at the end of Period 2 and Period 3 is that the atoms have 8 electrons in their outermost shell.
- If an element is in Group 17, it is likely to be non-metallic in character, while with one electron in its outermost energy level (shell), then it is likely to be metallic.
- In Period 3, the most metallic element is sodium.
Solution 20
(i) The properties of the elements are a periodic function of their atomic number.
(ii) Moving across a period of the periodic table, the elements show increasing non-metallic character.
(iii) The elements at the bottom of a group would be expected to show more metallic character than the elements at the top.
(iv) The similarities in the properties of a group of elements are because they have the same number of outer electrons.
Solution 21(i)
An anion is formed by the gain of electrons. In the chloride ion, the number of electrons is more than the number of protons. The effective positive charge in the nucleus is less, so the less inward pull is experienced. Hence, the size expands.
![]()
Solution 21(ii)
The inert gas argon is the next element after chlorine in the third period.
In a period, the size of an atom decreases from left to right due to an increase in nuclear charge with an increase in the atomic number. However, the size of the atoms of inert gases is bigger than the previous atom of halogen in the respective period. This is because the outer shell of inert gases is complete. They have the maximum number of electrons in their outermost orbit; thus, electronic repulsions are maximum. Hence, the size of the atom of an inert gas is bigger.
Solution 21(iii)
Ionisation potential of the element increases across a period because the atomic size decreases due to an increase in the nuclear charge, and thus, more energy is required to remove the electron(s).
Solution 21(iv)
To attain stability, atoms need to complete their octet by sharing, losing or gaining electrons. In inert gases, the octet is complete, and they do not need to gain, lose or share electrons. Hence, inert gas elements do not form ions.
Periodic Table, Periodic Properties and Variations of Properties Exercise Intext 3
Solution 1
(a) The energy required to remove an electron from a neutral isolated gaseous atom amd convert it into a positively charged gaseous ion is called Ionization energy or ionization potential.
(b) M(g)+ I.E ![]() M+(g)+ e-
M+(g)+ e-
M can be any element
It is measured in electron volts per atom. It's S.I unit kJmol-1.
Solution 2
Ionisation potential values depend on
a. Atomic size: The greater the atomic size, the lesser the force of attraction. Electrons of the outermost shell lie further away from the nucleus, so their removal is easier and the ionisation energy required is less.
b. Nuclear charge: The greater the nuclear charge, greater is the attraction for the electrons of the outermost shell. Therefore, the electrons in the outermost shell are more firmly held because of which greater energy is required to remove them.
Solution 3
(a) Ionization energy increases as we move from left to right across a period as the atomic size decreases.
(b) Ionization energy decreases down a group as the atomic size increases.
Solution 4
Helium has the highest ionization energy of all the elements while Sodium has the lowest ionization energy in first three periods.
Solution 5 (a)
Second period: Lithium < Beryllium < Boron < Carbon < Nitrogen < Oxygen < Fluorine < Neon
Third Period:Sodium < Magnesium < Aluminum < Silicon < Phosphorus < Sulphur < Chlorine < Argon
Solution 5 (b)
Correct option: D) Helium
Helium has highest ionisation potential 2372.0 kJ/mol.
Solution 6 (a)
Electron affinity is the energy released when a neutral gaseous atom acquires an electron to form an anion.
Unit of electron affinity: KJ/mol
Solution 7
Electron affinity values generally increase across the period left to right and decrease down the group top to bottom.
Solution 8(a)
Electronegativity is the tendency of an atom in a molecule to attract the shared pair of electrons towards itself.
Electronegativity is a dimensionless property; hence, it has no unit.
Solution 8(b)
Correct option - (i).
The element with least electronegativity is lithium.
Solution 8(c)
(ii) Chlorine
Solution 9
(a) On moving across a period, nuclear pull increases because of the increase in atomic number, and thus, the atomic size decreases. Hence, elements cannot lose electrons easily. Hence, Group 17 elements are strong non-metals, while Group 1 elements are strong metals.
(b) On moving across a period, nuclear pull increases because of the increase in atomic number, and thus, the atomic size decreases. Hence, elements cannot lose electrons easily. Hence, Group 17 elements are strong non-metals, while Group 1 elements are strong metals. Down a group, the atomic size increases and the nuclear charge also increases. The effect of an increased atomic size is greater as compared to the increased nuclear charge. Therefore, metallic nature increases as one moves down a group, i.e. they can lose electrons easily.
(c) The atomic size of halogens is very small. The smaller the atomic size, the greater the electron affinity, because the effective attractive force between the nucleus and the valence electrons is greater in smaller atoms, and so the electrons are held firmly.
(d) The reducing property depends on the ionisation potential and electron affinity of the elements. In a period, from left to right in a horizontal row of the periodic table, the atomic size decreases and the nuclear charge increases, so the electron affinity and ionisation energy both increase. Hence, the tendency to lose electrons decreases across the period from left to right and thus the reducing property also decreases across the period from left to right.
The electron affinity and ionisation potential decrease along the group from top to bottom. Hence, the tendency to lose electrons increases, and thus, the reducing property also increases along the group from top to bottom.
(e) In a period, the size of an atom decreases from left to right. This is because the nuclear charge, i.e. the atomic number increases from left to right in the same period, thereby bringing the outermost shell closer to the nucleus. Therefore, considering the third period given above, it has been found that sodium is the largest in size, while chlorine is the smallest.
Solution 10
(a) Ionization energy
(b) Metallic character
(c) Electronegativity
Solution 11
(a) G (due to the smallest atomic size).
(b) G and O as both have outermost electronic configuration np5.
(c) A and I as both have outermost electronic configuration ns1.
(d) D (1s22s22p2)
(e) I as alkali metals have least ionisation energy. Also, ionisation energy decreases with an increase in the atomic size that decreases on moving down the group.
(f) O, as halogens have the least atomic size.
Solution 12
(a) Thallium. Because the metallic character increases down the group, thallium will have the most metallic character.
(b) Boron. Electronegativity decreases down the group as the size increases; hence, boron will be the most electronegative atom.
(c) Three. The number of electrons present in the valence shell is the same for each group. Hence, all these elements and thallium will have 3 valence electrons.
(d) BCl3
(e) Since metallic character decreases from left to right and non-metallic character increases from left to right, elements in the group to the right of this boron group will be less metallic in character.
Periodic Table, Periodic Properties and Variations of Properties Exercise Ex. 1
Solution A 1
In the periodic table, alkali metals are placed in Group I. So, the correct option is A.
Solution A 2
The correct option is C.
The elements of halogen family are non-metallic in nature.
Solution A 3
B.
As we go from left to right in a period electrons are added to the same valence shell. Due to this atomic size decreases and nuclear charge increases. As nuclear charge increases, the energy required to remove an electron increases and hence ionization potential increases across a period.
Solution A 4
Correct option: (D) Argon
Solution A 5
The number of electrons in the valence shell of a halogen is 7.
Correct option: D
Solution A 6
Correct option: (D) Fluorine
Solution A 7
Correct option: D (atomic radius decreases and nuclear charge increases)
Solution A 8
Correct option: A (3 shells and 2 valence electrons)
Solution A 9
(i) Lithium
Reason: Electronegativity increases from left to right. Lithium is present on the left side of the periodic table; hence, it will be the least electronegative element.
Solution A 10
(A) 17
Element with atomic number 19 will lose 1 electron (to achieve the noble gas configuration) which can be accepted by the element with atomic number 17.
Solution A 11
(i) Increases
(ii) Increases
(iii) Increases
(iv) Decreases
(v) Increases
Solution A 12
Correct option: (c) ionic radius will increase
Solution:
While going down the halogen group of the periodic table, reactivity decreases, electronegativity decreases and ionization potential also decreases, but atomic and ionic radius increases due to the addition of a new shell of electrons.
Solution A 13
Correct option: (b) Energy released when an electron is added to an isolated atom in the gaseous state.
Solution:
The energy released in the process of adding an electron to a neutral gaseous isolated atom and forming a negatively charged ion is termed electron affinity.
Solution A 14
Correct option: (d) Reactivity increases in alkali metals but decreases in halogens.
Solution:
The reactivity of alkali metals (group 1) increases down the group because as the atomic radius increases in size with an increase in shells of electrons, the effective nuclear charge experienced by valence shell electrons decreases. Hence, Francium can easily donate electrons forming a cation than lithium. The reactivity of the halogens (Group 17) decreases down the group because the atomic radius increases in size with an increase in shells of electrons. This lessens the attraction for valence electrons of other atoms, decreasing reactivity.
Solution A 15
Correct option: (c) Sr, Ca, Mg, Be
Solution:
In group 2 (alkaline earth metals), the ionization energy of Be will be highest due to having the smallest atomic size followed by Mg, Ca, and Sr moving down the group.
Solution B 1(a)
The element below sodium in the same group would be expected to have a lower electro-negativity than sodium, and the element above chlorine would be expected to have a higher ionisation potential than chlorine.
Solution B 1(b)
On moving from left to right in a given period, the number of shells remains the same.
Solution B 1(c)
On moving down a group, the number of valence electrons remains the same.
Solution B 1(d)
Metals are good reducing agents because they are electron donors.
Solution B 1(e)
Across a period, the ionisation potential increases.
Solution B 1(f)
Down the group, electron affinity decreases.
Solution B 1(g)
Electronegativity across the period increases.
Solution B 1(h)
Non-metallic character down the group decreases.
Solution B 1(i)
In a period, increase in electron affinity increases reduction.
Solution B 1(j)
On descending a group decrease in ionization potential as well as electron affinity decrease oxidizing capacity.
Solution B 1(k)
Metals are good reducing agents because they are electron donors.
Solution B 1(l)
If an element has a low ionization energy then it is likely to be metallic.
Solution B 1(m)
If an element has seven electrons in its outermost shell then it is likely to have the Smallest atomic size among all the elements in the same period.
Solution B 2
1. less than
2. less than
Solution B 3
i. The element B would have higher metallic character than A.
ii. The element A would probably have higher electron affinity than B.
iii. The element A would have smaller atomic size than B.
Solution B 4
Electronic configuration of E with atomic number 19 = 1s22s22p63s23p64s1
E is a metal.
Electronic configuration of F with atomic number 8 =
1s22s22p4
F is a non-metal.
Electronic configuration of G with atomic number 17 =
1s22s22p63s23p5
G is a non-metal.
Solution B 5
- Mg, Cl, Na, S, Si (decreasing order of atomic size) -
Na > Mg > Si > Cl
186 pm > 160 pm > 117pm > 104pm > 99pm
- Cs, Na, Li, K, Rb (increasing metallic character)
Li < Na < K < Rb < Cs - Na, Mg, Cl, S, Si (increasing ionisation potential) -
Na < Mg < Si < S < Cl
495.8 ev < 737.7 ev < 786.5 ev < 999.6 ev < 1251.2 ev - Cl, F, Br, I (increasing electron affinity) -
I < Br < F < Cl
-295 KJ mol-1 < -324 KJ mol-1 < -327.9 KJ mol-1 < -349 KJ mol-1 - Cs, Na, Li, K, Rb (decreasing electronegativity) -
Li > Na > K = Rb > Cs
> 0.9 > 0.8 = 0.8 > 0.7 - Pb < Zn < Ca < K (According to the position in reactivity series)
- H > Li > Na > K (Smaller the size greater is the ionization potential)
Solution B 6
Na, Mg, Al, Si, P, S, Cl
Solution B 7
A metal present in Period 3, Group I of the periodic table is sodium.
Solution B 8
Electron affinity
Solution B 9
2nd
Solution B 10
Three shells indicate that the element belongs to the third period.
Three valence electrons indicate that the element belongs to the third group.
Solution C 1
Elements are arranged in the periodic table in the increasing order of their atomic number.
It states that the properties of elements are the periodic functions of their atomic number i.e. if the elements are arranged in tabular form in the increasing order of their atomic numbers then the properties of the elements are repeated after definite regular intervals or periods.
Solution C 2(i)
(a) Cl < Cl¯
(b) Mg2+ < Mg+ < Mg
(c) O < N < P
Solution C 2(ii)
(a) Cl
Metals have low ionisation energy and non-metals have high ionisation energy. Also, across the period, ionisation energy tends to increase. The elements P, Na and Cl belong to the third period. Na - Group 1, P - Group 15 and Cl - Group 17.
(b) Ne
Inert gases have zero electron affinity because of their stable electronic configuration.
(c) He
Ionisation energy decreases with an increase in the atomic size, i.e. it decreases as one moves down a group. Ne, He and Ar are inert gases. He - Period 1, Ne - Period 2 and Ar - Period 3.
Solution C 3
These elements will form anions.
Explanation:
These elements belongs to VIA (16), VA(15), IA(1), IIA(2), IIIA(13) and VIIA(17)groups generally these elements form anion.
Solution C 4(a)
The oxidising power of elements depends on the tendency to gain electrons which increases from left to right along a period due to increase in nuclear pull.
Solution C 4(b)
Because the atomic radius decreases across a period. Due to this, attraction between the nucleus and the electron increases. This results in an increase in the ionisation potential.
Solution C 4(c)
Alkali metals are good reducing agents because they have a greater tendency to lose electrons.
Solution D 1
(a) An element with atomic number 9 and 35
(b) An element with atomic number 9.
Solution D 2
The ionisation energy is the minimum energy required to remove the outermost electron from a gaseous neutral atom to form a cation.
Position in a group: X will be above Y ( because of ionisation energy decreases down the group )
Position in a period: X will be the right side of Y ( because ionisation energy increases from left to right)
Solution D 3
(a) Period 2
(b) Nitrogen (N), between carbon and oxygen
(c) Be< N< F
(d) Fluorine
Solution D 4
The atomic number of an element Z is 16. Therefore,
(a) It belongs to Period 3.
(b) The number of valence electrons in the element is 6.
(c) The element is a non-metal.
(d) The formula between the hypothetical element Z and hydrogen would be H2Z. More specifically as we know that atomic number of sulphur is 16, so two hydrogen atoms combine with one sulphur atom to form hydrogen sulphide (H2S) gas.
Solution D 5
(a) Ionic bond exists between M and O.
(b) 1 electron is present in the outermost shell of M.
(c) M belongs to Group 1 in the modern periodic table.
Solution D 6
(a) Ba metal will form ions readily because the ionisationenergy decreases down the group as the size increases.
(b) On moving down the group, the number of electrons in the outermost shell, i.e.valence electrons remain the same. So, the valency in a group remains the same, i.e. 2.
Solution D 7
(a) Element B forms an electrovalent compound with G.
(b) The ion of element B (B2+) will migrate towards the cathode during electrolysis.
(c) The non-metallic element which has the valency of 2 is E.
(d) F is an inert gas.
Solution D 8
(i) The most electronegative element is J.
(ii) The most reactive element of group IA or 1 is B.
(iii) The element from period 3 with the smallest atomic size is K.
(iv) The element with the highest ionization potential is Li.
(v) The number of valence electrons present in G is 5.
(Disclaimer: Q is absent in the given table, so calculated for G)
(vi) The element from group 2 that will have the least
ionization energy is C.
(vii) The noble gas of the fourth period is Krypton.
(Disclaimer: The fourth period is not given in the table so given the actual name of the noble gas from the periodic table).
(viii) A2H
(ix) The electron dot structure for the compound formed between C and K is: