Class 10 SELINA Solutions Chemistry Chapter 3 - Acids, Bases and Salts
Acids, Bases and Salts Exercise Intext 1
Solution 1
(a) Acids are defined as compounds which contain one or more hydrogen atoms, and when dissolved in water, they produce hydronium ions (H3O+), the only positively charged ions.
(b) Hydronium ion
(c) H3O+
Solution 2
H2SO4 + H2O ⇌ H3O+ + HSO4-
HSO4- + H2O ⇌ H3O+ + SO4-2
Solution 3
If water is added to a concentrated acid, the heat generated causes the mixture to splash out and cause severe burns. Thus, water is never added to acid in order to dilute it.
Solution 4
Basicity: The basicity of an acid is defined as the number of hydronium ions (H3O+) that can be produced by the ionization of one molecule of that acid in aqueous solution.
The basicity of following compounds are:
Nitric acid:Basicity= 1
Sulphuric acid: Basicity=2
Phosphoric acid: Basicity=3
Solution 5
(a) Oxyacids: - HNO3, H2SO4
(b) Hydracid:- HCl, HBr
(c) Tribasic acid:- H3PO4, H3PO3
(d) Dibasic acid: - H2SO4 , H2CO3
Solution 6
(a) The anhydride of following acids are:
(i) Sulphurous acid: SO2
(ii) Nitric acid: N2O5
(iii) Phosphoric acid: P2O5
(iv) Carbonic acid: CO2
(b) Acids present in following are:
Vinegar: Acetic acid
Grapes: Tartaric acid and Malic acid
Lemon: Citric acid
Solution 7
Acetic acid is a monobasic acid which on ionization in water produce one hydronium ion per molecule of the acid.
Solution 8
(i) 2NO2(g) + H2O(l)→ HNO2(aq) + HNO3
(ii) H2S2O7 + H2O → 2 H2SO4
Solution 9
The strength of an acid is the extent to which the acid ionizes or dissociates in water.
The strength of an acid depends on the degree of ionization and concentration of hydronium ions [H3O+] produced by that acid in aqueous solution.
Solution 10
(a)Carbonic acid is a dibasic acid with two replaceable hydrogen ions; therefore it forms one acid salt or one normal salt.
Hydrochloric acid is a monobasic acid with one replaceable hydrogen ion and so forms only one normal salt.
(b) Strength of an acid is the measure of concentration of hydronium ions it produces in its aqueous solution. Dil. HCl produces high concentration of hydronium ion compared to that of concentrated acetic acid. Thus, dil. HCl is stronger acid than highly concentrated acetic acid.
(c) H3PO3 is not a tribasic acid because in oxyacids of phosphorus, hydrogen atoms which are attached to oxygen atoms are replaceable. Hydrogen atoms directly bonded to phosphorus atoms are not replaceable.
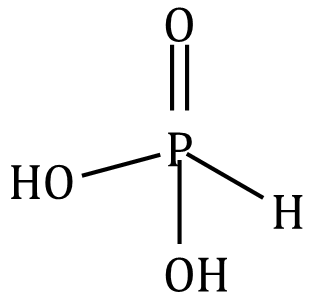
(d) The salt produced is insoluble in the solution so the reaction does not proceed. Hence, we do not expect lead carbonate to react with hydrochloric acid.
(e) NO2 is called double acid anhydride because two acids – nitrous acid and nitric acid – are formed when it reacts with water.
2NO2 + H2O → HNO2 + HNO3
Solution 11
(a) Acids are prepared from non-metals by their oxidation. For example :
Sulphur or phosphorus is oxidized by conc. Nitric acid to form sulphuric acid or phosphoric acid.
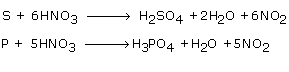
(b) Acids are prepared from salt by the displacement reaction. For example :
Nitric acid is prepared by using H2SO4 and sodium chloride.
![]()
Solution 12
(a) SO2 +H2O ![]() H2SO3
H2SO3
(b) P2O5 +3H2O ![]() 2H3PO4
2H3PO4
(c) CO2 + H2O ![]() H2CO3
H2CO3
(d)![]()
Solution 13
(a) Citric acid
(b) Carbonic acid
(c) Oxalic acid
(d) Boric acid
Solution 14
(a) Chlorides react with concentrated sulphuric acid on warming to liberate hydrogen chloride.
![]()
(b) Nitrates when heated with conc. sulphuric acidproduce more volatile nitric acid.
![]()
Both chlorides and nitrates do not react with dilute acids; they react with concentrated acids.
Acids, Bases and Salts Exercise Ex. 3(A)
Solution 1
An alkali is a basic hydroxide which when dissolved in water produces hydroxyl ions (OH-) as the only negatively charged ions.
(a) Strong alkalis: Sodium hydroxide , Potassium hydroxide
(b) Weak alkalis: Calcium hydroxide , Ammonium hydroxide
Solution 2
(a) An alkali and a base:
1. Alkalis are soluble in water whereas bases may be or may not be soluble in water.
2. All alkalis are bases but all bases are not alkalis.
(b) The chemical nature of an aqueous solution of HCl and an aqueous solution of NH3
1. The aqueous solution of HCl is acidic in nature. It can turn blue litmus to red.
2. The aqueous solution of NH3 is basic in nature. It can turn red litmus to blue.
Solution 3
a. Hydroxyl ion (OH-)
b. H+
Solution 4
(a) Barium oxide
(b) Sodium hydroxide
(c) Manganese oxide
(d) Cupper hydroxide
(e) Carbonic acid
(f) Ferric hydroxide
(g) Copper oxide
(h) Ammonia
(i) Ammonium hydroxide
Solution 5
The test tube containing distilled water does not affect the red litmus paper.
The test tube containing acidic solution does not change the red litmus paper.
But the test tube containing basic solution turns red litmus paper blue.
Solution 6
It is because HCl and HNO3 ionize in aqueous solution whereas ethanol and glucose do not ionize in aqueous solution.
Solution 7
a. Dry HCl gas does not contain any hydrogen ions in it, so it does not show acidic behaviour. Hence, dry HCl gas does not change the colour of dry litmus paper.
b. Lead oxide is a metallic oxide which reacts with hydrochloric acid to produce lead chloride and water, but it is excluded from the class of bases, because chlorine is also produced.
PbO2 + 4HCl → PbCl2 + Cl2 + 2H2O
Thus, lead oxide is not a base.
c. Yes, basic solutions have H+ ions, but the concentration of OH- ions is more than the H+ ions which makes the solution basic in nature.
Solution 8
(a) We can obtain a base from another base by double decomposition. The aqueous solution of salts with base precipitates the respective metallic hydroxide.
FeCl3 +3NaOH ![]() Fe(OH)3 +3NaCl
Fe(OH)3 +3NaCl
(b) An alkali from a base
![]()
(c) Salt from another salt
![]()
Solution 9
(a) Mg +2HCl ![]() MgCl2 + H2
MgCl2 + H2
(b) HCl + NaOH ![]() NaCl + H2O
NaCl + H2O
(c) CaCO3 +2HCl ![]() CaCl2 +H2O + CO2
CaCl2 +H2O + CO2
(d) CaSO3 + 2HCl ![]() CaCl2 + H2O+ SO2
CaCl2 + H2O+ SO2
(e) ZnS + 2HCl ![]() ZnCl2 + H2S
ZnCl2 + H2S
Solution 10
As we know that alkalis react with oil to form soap. As our skin contains oil so when we touch strong alkalis, a reaction takes place and soapy solution is formed. Hence we should wear gloves.
Solution 11
|
Indicator |
Neutral |
Acidic |
Alkaline |
|
Litmus Phenolphthalein |
Purple Colourless |
Blue to red Colourless |
Red to blue Pink |
Solution 12
pH represents the strength of acids and alkalis expressed in terms of hydrogen ion concentration.
The solution with pH value 10 will give pink colour with phenolphthalein indicator.
Solution 13
A = Strongly acidic
B= neutral
C=Weakly alkaline
D= Strongly alkaline
E= Weakly acidic
(a) Solution A (acidic solution) + Mg![]() H2 + Mg salt
H2 + Mg salt
(b) SolutionA (acidic solution) + Zn![]() H2 + Zn salt
H2 + Zn salt
Solution 14
(a) A common acid-base indicator and a universal indicator:
An acid-base indicator like litmus tells us only whether a given substance is an acid or a base. The universal indicator gives an idea as to how acidic or basic a substance is universal indicator gives different colours with solutions of different pH values.
(b) The acidity of bases and basicity of acids
The acidity of bases: The number of hydroxyl ions which can be produced per molecule of the base in aqueous solution.
Basicity of acid: The basicity of an acid is defined as the number of hydronium ions that can be produced by the ionization of one molecule of that acid in aqueous solution.
(c) Acid and alkali:
An acid is that substance which gives H+ ions when dissolved in water.
An alkali is that substance which gives OH- ions when dissolved in water.
Solution 15
(a) Alkali
(b) Acid
Solution 16
Substances like chocolates and sweets are degraded by bacteria present in our mouth. When the pH falls to 5.5 tooth decay starts. Tooth enamel is the hardest substance in our body and it gets corroded. The saliva produced by salivary glands is slightly alkaline, it helps to increase the pH, to some extent, but toothpaste which contains basic substance is used to neutralize excess acid in the mouth.
Solution 17
A universal indicator is a mixture of dyes which identify a gradual change of various colours over a wide range of pH, depending on the strength of the acid. When we use a universal indicator, we see solutions of different acids produce different colours. Indeed, solutions of the same acid with different concentration give different colours.
The more acidic solutions turn universal indicator bright red. A less acidic solution will only turn it orange-yellow. Colour differences can also be observed in case of vinegar which is less acidic and battery acid which is more acidic.
Solution 18
a.
i. The pH can be increased by adding a basic solution.
ii. The pH can be increased by adding an acidic solution.
b. The solution is basic in nature and the pH value will be more than 7.
c. Less than 7
Solution 19
a. Solution P
b. Solution R
c. Solution Q
Solution 20
(a) blue
(b) red
(c) hydrogen gas
(d) basic, alkaline
(e) graphite
Acids, Bases and Salts Exercise Ex. 3(B)
Solution 1
(a) A normal salt: Normal salts are the salts formed by the complete replacement of the ionisable hydrogen atoms of an acid by a metallic or an ammonium ion.
(b) An acidic salt: Acid salts are formed by the partial replacement of the ionisable hydrogen atoms of a polybasic acid by a metal or an ammonium ion.
(c) A basic salt: Basic salts are formed by the partial replacement of the hydroxyl group of a di- or tri- acidic base by an acid radical.
Examples:
(a) A Normal salt: Na2SO4, NaCl
(b) An acid salt: NaHSO4, Na2HPO4
(c) A basic salt: [Pb(OH)Cl], [Mg(OH)Cl].
Solution 2
(a) Salt is a compound formed by the partial or total replacement of the ionizable hydrogen atoms of an acid by a metallic ion or an ammonium ion.
(b) An insoluble salt can be prepared by precipitation.
(c) A salt prepared by direct combination is Iron(III) chloride.
Reaction:
2Fe +3Cl2 ![]() 2FeCl3
2FeCl3
(d) By neutralizing sodium carbonate or sodium hydroxide with dilute sulphuric acid:
Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2
2 NaOH + H2SO4 → Na2SO4 + 2H2O
Solution 3
(a) Direct combination
When two substance reacts chemically to form a new substance which is a compound it is a direct combination reaction. This reaction is also termed as synthesis.
This reaction taken place when two elements are heated together.
Metal + Non metal → Salt
For example,
2Na(molten) + Cl2 → 2NaCl
(b) Displacement:
Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound.
For example,
Action of dilute acids on active metals. Soluble salts of active metals are prepared by this method.
Active metal + Acid (dil.) → Salt + Hydrogen
Zn + H2SO4 → ZnSO4 + H2↑
(c) Double decomposition (precipitation):
It is a chemical change, in which two compounds in solution react to form two other compounds by the mutual exchange of radicals. A solid precipitate is formed as a result of the reaction.
For example,
Barium is more reactive than hydrogen, hence it displaces hydrogen from its compound H2SO4 in aqueous solution to form barium sulphate precipitate. Hence, this reaction is also called as precipitation reaction. The chemical reaction is as follows:
BaCl2 + H2SO4![]() BaSO4 + 2HCl
BaSO4 + 2HCl
(d) Neutralisation of insoluble base:
Acid + Insoluble base → Salt + Water
Preparation of copper (II) sulphate (or blue vitriol)
By the action of dilute acid (dil. H2SO4) on an insoluble base copper hydroxide, copper oxide or copper carbonate.
For example,
Cu(OH)2 + H2SO4 → CuSO4 + 2H2O
(e) Neutralisation of an alkali (also called as titration):
Acid + Soluble base → Salt + Water
For example,
Neutralisation of caustic soda with dilute sulphuric acid.
Reaction:
2NaOH + H2SO4 → Na2SO4 + 2H2O
This method is useful for preparing the soluble salts of only sodium, potassium and ammonium. Since, the reactants as well as the products are soluble, a titration is conducted to determine the completion of the neutralization reactions.
Solution 4
(a) Copper sulphate crystals from a mixture of charcoal and black copper oxide:
The carbon in the charcoal reduces the black copper oxide to reddish-brown copper. The lid must not be removed until the crucible is cool or the hot copper will be re-oxidized by air.
Take dilute sulphuric acid in a beaker and heat it on wire gauze. Add cupric oxide in small quantities at a time, with stirring till no more of it dissolves and the excess compound settles to the bottom.
Filter it hot and collect the filtrate in a china dish. Evaporate the filtrate by heating to the point of crystallization and then allow it to cool and collect the crystals of copper sulphate pentahydrate.
Reaction: CuO + H2SO4 ![]() CuSO4 + H2O
CuSO4 + H2O
CuSO4 + 5H2O ![]() CuSO4. 5H2O
CuSO4. 5H2O
(b) Zinc sulphate crystals from Zinc dust:
Take dilute sulphuric acid in a beaker and heat it on wire gauze. Add some granulated zinc pieces with constant stirring. Add till the Zinc settles at the base of the beaker. Effervescences take place because of the liberation of hydrogen gas. When effervescence stops, it indicates that all the acid has been used up. The excess of zinc is filtered off. Collect the solution in a china dish and evaporate the solution to get crystals. Filter, wash them with water and dry them between the folds of paper. The white needle crystals are of hydrated Zinc sulphate.
Reaction:Zn + H2SO4 ![]() ZnSO4 + H2
ZnSO4 + H2
ZnSO4 +7 H2O![]() ZnSO4. 7 H2O
ZnSO4. 7 H2O
(c) Lead sulphate from metallic lead:
Metallic lead is converted to lead oxide by oxidation. Then lead sulphate is prepared from insoluble lead oxide, by first converting it into soluble lead nitrate. Then the lead nitrate solution is treated with sulphuric acid to obtain white ppt. of Lead sulphate.
Reaction:
PbO +2HNO3 ![]() Pb(NO3)2 + H2O
Pb(NO3)2 + H2O
Pb(NO3)2 + H2SO4 ![]() PbSO4 + 2HNO3
PbSO4 + 2HNO3
(d)Sodium hydrogen carbonate crystals:
Dissolve 5 grams of anhydrous sodium carbonate in about 25 ml of distilled water in a flask. Cool the solution by keeping the flask in a freezing mixture. Pass carbon dioxide gas in the solution. Crystals of sodium bicarbonate will precipitate out after some time. Filter the crystals and dry it in folds of filter paper.
Reaction: Na2CO3 + CO2 + H2O ![]() 2NaHCO3
2NaHCO3
Solution 5
1. Anhydrous ferric chloride: -A (Direct combination of two elements)
2Fe + 3Cl2![]() 2FeCl3
2FeCl3
2.Lead chloride: -E (Reaction of two solutions of salts to form a precipitate)
Pb(NO3)2 +2HCl ![]() PbCl2 +2HNO3
PbCl2 +2HNO3
3.Sodium sulphate: - D( Titration of dilute acid with a solution of soluble base)
2NaOH + H2SO4 ![]() Na2SO4 +2H2O
Na2SO4 +2H2O
4. Copper sulphate:- C (reaction of dilute acid with an insoluble base)
Cu(OH)2 +H2SO4 ![]() CuSO4 + 2H2O
CuSO4 + 2H2O
Solution 6
(a) Lead chloride
(b) Silver chloride
(c) Barium sulphate and lead sulphate
(d) Basic lead chloride
(e) Sodium hydrogen sulphate
(f) Sodium potassium carbonate
(g) Sodium argentocyanide
(h) Potash alum
Solution 7
An acid is a compound which when dissolved in water forms hydronium ions as the only positively charged ions. A base is a compound which is soluble in water contains hydroxide ions. A base reacts with an acid to form a salt and water only. This type of reaction is known as neutralisation.
Solution 8
(a) Blue litmus will turn into red which will indicate the solution to be acidic.
(b) No change will be observed.
(c) Red litmus will turn into blue will indicate the solution to be basic.
Solution 9
(a) Since sodium hydroxide and sulphuric acid are both soluble, an excess of either of them cannot be removed by filtration. Therefore it is necessary to find out on small scale, the ratio of solutions of the two reactants.
(b) As iron chloride is highly deliquescent, so it is kept dry with the help of fused calcium chloride.
(c) On heating the hydrate, HCl acid is released and basic salt (FeOCl) or ferric oxide remains. Hence, anhydrous ferric chloride cannot be prepared by heating the hydrate.
Solution 10
Zinc Sulphate - Displacement
![]()
Ferrous sulphide - synthesis
![]()
Barium sulphate - Precipitation
![]()
Ferric Sulphate- Oxidation
![]()
Sodium sulphate - Neutralisation
![]()
Solution 11
(a) pH of pure water is 7 at 25oC. No, the pH does not change when common salt is added.
(b) Acids: H2SO4 and HNO3
Bases: Ammonium hydroxide and sodium hydroxide.
Salts: Barium chloride and sodium chloride.
Solution 12
|
Reactants |
Products |
Method |
|
Soluble base + Acid (dil) |
Salt + water |
Neutralisation Titration |
|
Metal + Non-metal |
Salt (soluble/insoluble) |
Direct Combination |
|
Insoluble base + |
Salt (soluble) + water |
……………. |
|
Active metal + Acid (dil) |
Salt + Hydrogen |
Displacement |
|
Soluble salt solution (A) + Soluble salt solution (B) |
Precipitated salt + Soluble salt |
Precipitation |
|
Carbonate /bicarbonate + Acid (dil) |
Salt + Water+ Carbon dioxide |
Decomposition of carbonate |
|
Chlorides/nitrates + Acid (conc) |
Acid salt + HCl/HNO3 |
Decomposition of chlorides and nitrates |
Solution 13
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
Solution 14
(a) Iron (III) Chloride: Iron chloride is formed by direct combination of elements.
![]()
(b) Sodium sulphate: By neutralization of caustic soda with dilute sulphuric acid
![]()
(c) Sodium zincate: By the action of metals with alkalis
![]()
(d) Iron (II) sulphate: Iron sulphate is prepared by the action of dilute acid on an active metal.
![]()
(e) Sodium chloride: By the neutralization reaction of strong acid with strong base
![]()
Solution 15
a. By neutralisation:
NaOH + HCl → NaCl + H2O
b. By precipitation:
Pb(NO3)2 + 2NaCl → PbCl2 + 2NaNO3
c. CuCO3 + H2SO4→ CuSO4 + H2O + CO2
d. Simple displacement:
Zn + H2SO4→ ZnSO4 + H2
Solution 16
a. Na2CO3 + H2SO4 (dil) → Na2SO4 + H2O + CO2
b. CuCO3 + H2SO4 (dil) → CuSO4 + H2O + CO2
c. Fe + H2SO4 (dil) → FeSO4 + H2
d. Zn + H2SO4 (dil) → ZnSO4 + H2
ZnSO4 + Na2CO3 → ZnCO3 + Na2SO4
Solution 17
a. NaHSO4
b. AgCl
c. CuSO4.5H2O
d. CuCO3
e. Pb(NO3)2
Solution 18
a. acid salt
b. NaOH + HCl → NaCl + H2O
Solution 19
a. Alkali
b. Precipitate
c. Weak acid
Solution 20
i. Copper (II) chloride - B
ii. Iron (II) chloride - A
iii. Iron (III) chloride - C
iv. Lead (II) chloride - E
v. Sodium chloride - D
Solution 21
- A covalent oxide of a metalloid - SiO2
- An oxide which when dissolved in water from acid- SO2
- A basic oxide- Na2O, MgO
- An amphoteric oxide- Al2O3
Acids, Bases and Salts Exercise Ex. 3(C)
Solution 1
It is the amount of water molecules which enter into loose chemical combination with one molecule of the substance on crystallisation from its aqueous solution.
|
Common name |
Chemical name |
Formula |
|
Washing soda |
Sodium carbonate decahydrate |
Na2CO3.10H2O |
|
Epsom salt |
Magnesium sulphate heptahydrate |
MgSO4. 7H2O |
|
Potash alum |
Hydrated potassium aluminium sulphate |
K2SO4.Al(SO4)3.24H2O |
|
Gypsum |
Hydrated calcium sulphate |
CaSO4.2H2O |
Solution 2
a. Crystalline hydrated salts which on exposure to the atmosphere lose their water of crystallisation partly or completely and change into a powder. This phenomenon is called efflorescent and the salts are called efflorescent.
Examples: CuSO4.5H2O, MgSO4.7H2O, Na2CO3.10H2O
b. Water-soluble salts which on exposure to the atmosphere absorb moisture from the atmosphere and dissolve in the same and change into a solution. The phenomenon is called deliquescence and the salts are called deliquescent.
Examples: CaCl2, MgCl2, ZnCl2
Solution 3
a. Water of crystallization
b. White
c. By heating with any dehydrating agent
d. A deliquescent salt – Calcium chloride, zinc chloride
e. Hydrated copper sulphate turns white on heating because, it loses water of crystallisation.
Solution 4
(a) When washing soda (Na2CO3.10H2O) is exposed to air, it loses 9 molecules of water to form a monohydrate.
![]()
(b) It absorbs moisture from the atmosphere and becomes moist and ultimately dissolves in the absorbed water, forming a saturated solution.
Solution 5
a. Sodium hydrogen sulphate [NaHSO4] is an acid salt and is formed by the partial replacement of the replaceable hydrogen ion in a dibasic acid [H2SO4]. The [H] atom in NaHSO4 makes it behave like an acid.
So, on dissolving in water, it gives hydrogen ions.
b. Desiccating agents are used to absorb moisture. Anhydrous calcium chloride (CaCl2) has the capacity of absorbing moisture as it is hygroscopic in nature. So, it is used in a desiccator.
Solution 6
Conc. sulphuric acid is hygroscopic in nature and can remove moisture from other substances; therefore, it is used as a drying agent.
It is also used as a dehydrating agent because it has a strong affinity for water and thus absorbs water quickly from compounds.
Solution 7
|
Drying agents |
Dehydrating agents |
|
They remove moisture from other substances. |
They remove chemically combined elements of water in the ratio of 2:1 (hydrogen:oxygen) from a compound. |
|
They are used to dry gases like chlorine, sulphur dioxide and hydrogen chloride. They are also used in dessicators to keep substances dry. |
They prepare substances such as carbon monoxide and sugar charcoal. |
|
They represent a physical change. |
They represent a chemical change. |
Solution 8
a. Increase
b. Increase
c. Decrease
d. Increase
e. Increase
Solution 9
a. Table salt turns moist and ultimately forms a solution on exposure to air especially during the rainy season. Although pure sodium chloride is not deliquescent, the commercial version of the salt contains impurities (such as magnesium chloride) which are deliquescent substances.
b. The impurity can be removed by passing a current of dry hydrogen chloride gas through a saturated solution of the affected salt. Pure sodium chloride is produced as a precipitate which can be recovered by filtering and washing first with a little water and finally with alcohol.
c. Conc. sulphuric acid
d. Common salt and sugar
Solution 10
(a) Iron chloride(FeCl3)
FeCl3 + 3H2O → 3HCl + Fe(OH)3
(b) Ammonium acetate (CH3COONH4)
CH3COONH4 +H2O → CH3COOH + NH4OH
(c) Sodium chloride
NaCl(s) + H2O → Na+(aq) OH-(aq) + H2O
Solution 11
(a) Na2CO3 solution: This solution is alkaline in nature; hence, red litmus changes to blue.
(b) NaCl solution: There is no change in the colour of the litmus paper because this solution is neutral.
(c) NH4NO3: This solution is alkaline in nature; hence, red litmus changes to blue.
(d) MgCl2: It is slightly acidic and neutral; hence, there is no change in the litmus paper.
Exercise Misc.
Solution A 1
(d) Acetic acid
Solution A 2
(d) Copper (II) oxide
Solution A 3
(a) Formic acid
Solution A 4
(d) Tetrammine copper [II] sulphate
Solution A 5
Correct option: (a) CO
From all these gases only carbon monoxide does not react with water to form an acid.
Solution A 6
Correct option: (b) 1
Solution B 1
i. Hydronium
ii. Hydroxide
iii. Salt
iv. Water
v. Hydrogen
Solution B 2
(i) C
(ii) A
(iii) E
(iv) B
(v) D
Solution B 3
|
Column I |
Column II |
|
Pb(NO3)2 from PbO |
Precipitation |
|
MgCl2 from Mg |
Simple displacement |
|
FeCl3 from Fe |
Combination |
|
NaNO3 from NaOH |
Neutralisation |
|
ZnCO3 from ZnSO4 |
Titration |
Solution B 4
SO2 is an acidic oxide which dissolves in water forming an acid. ![]()
Solution B 5
(i) B
(ii) D
(iii) E
Solution B 6
|
a deliquescent salt |
an insoluble chloride |
|
MgCl2 |
AgCl |
Solution B 7
(i) Acidic salt
(ii) Water of crystallisation
(iii) Deliquescence
(iv) Complex salts
(v) Alkali
Solution B 8
(i) (B) Neutralisation
(ii) (E) Direct synthesis
(iii) (D) Double decomposition
(iv) (A) Simple displacement
(v) (C) Decomposition by acid
Solution C 1
A neutralisation reaction is when an acid and a base react to form water and a salt, and involves the combination of H+ ions and OH- ions to generate water.
Solution C 2
Zinc granule is added to copper sulphate solution:
When zinc granules are added to the solution of copper sulphate, zinc displaces copper to form zinc sulphate and thus copper gets deposited. In this reaction, zinc metal can easily displace the copper metal from its salt to form an aqueous solution of zinc sulphate. This is an example of displacement reaction.
Zn(s) + CuSO4 (aq) → ZnSO4 (aq) + Cu↓
Solution C 3
Salt S is prepared by reacting dilute sulphuric acid with copper oxide. Hence, salt S is copper sulphate.
Solution C 4
(i) ![]()
(ii) ![]()
(iii) ![]()
(iv) ![]()
![]()
Solution C 5
Chemical equations:
(a) Sodium sulphate:
2Na + H2SO4 (dil.) → Na2SO4 + H2↑
(b) Zinc carbonate:
Zn(NO3)2 + CuCO3→ ZnCO3 + Cu(NO3)2
(c) Copper (II) sulphate:
CuCO3 + H2SO4 (dil.) → CuSO4 + H2O + CO2
(d) Iron (II) sulphate:
Fe + H2SO4 (dil.) → FeSO4 + H2↑
Solution C 6
(a) Solution B
(b) Solution C
Solution C 7(a)
Silver nitrate solution with sodium chloride solution:
When Silver nitrate (AgNO3) is mixed with the solution of Sodium chloride (NaCl) then the ions of both the compounds get exchanged. As a result, white precipitates of Silver chloride is formed (AgCl) and the solution of Sodium nitrate (NaNO3) are formed. This is an example of double displacement reaction.
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3
Solution C 7(b)
Pb(NO3)2 + 2NaCl → PbCl2 + 2NaNO3
Solution C 7(c)
When crystals of washing soda are exposed to air, it loses its water of crystallisation and the phenomenon is known as efflorescence.
Solution C 8
(a)
![]()
(b) ![]()
(c)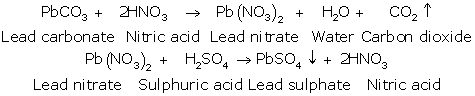
(d) ![]()
(e)![]()
![]()
(f)![]()
(g)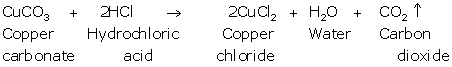
Solution C 9
(a) Fe + 2HCl (dil) ![]() FeCl2 + H2
FeCl2 + H2
(bi) 2Fe (heated) + 3Cl2 (dry) ![]() 2FeCl3
2FeCl3
(c) Fe + H2SO4 (dil) ![]() FeSO4 + H2
FeSO4 + H2
(d) Fe + S ![]() FeS
FeS
Solution D 1
The stable positive ion formed when an acid dissolves in water is hydronium ion. The structure of hydronium ion (H3O+) is as follows:
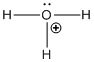
Solution D 2
A = HCl
B = Na2CO3
C = HNO3
D = NaOH
A: Fe + 2HCl → FeCl2 + H2
B: FeCl2 + Zn→ ZnCl2 + Fe
Fe + H2CO3 → FeCO3 + H2↑
C: FeCO3 + 2HNO3 → Fe (NO3)2 + H2O + CO2
D: Fe(NO3)2 + 2NaOH → Fe(OH)2 + 2NaNO3
Solution D 3
i. ![]()
ii. ![]()
iii. ![]()
![]()
iv. ![]()
![]()
Solution D 4
(a) The first step is to convert insoluble lead carbonate into soluble lead nitrate by treating lead carbonate with dilute nitric acid.
(b) PbCO3 (s) + 2HNO3(dil) ![]() Pb(NO3)2 (aq) + H2O (l) + CO2
Pb(NO3)2 (aq) + H2O (l) + CO2 ![]()
(c) When dilute sulphuric acid is added directly to lead carbonate, the lead sulphate thus formed will be deposited on solid lead carbonate disconnecting lead carbonate from sulphuric acid.
Solution D 5
(i) B is an anhydrous calcium chloride.
(ii) B absorbs moisture from the receiver.
(iii) Because iron (III) chloride is highly deliquescent and it absorbs moisture from the surrounding air to form a saturated solution.
(iv)
![]()

