Class 11-science NCERT Solutions Biology Chapter 9 - Biomolecules
Biomolecules Exercise 119
Solution 1
The biomolecules formed by the polymerisation of a large number of micromolecules with higher molecular weight are known as macromolecules. These occur in colloidal state in intracellular fluid as they are insoluble in nature. Examples: Proteins and polysaccharides
Solution 2
The tertiary structure is a structure formed by the folding of secondary coiled polypeptides forming a hollow, woollen ball-like structure. It is folded in such a way that the functional side groups are present on the surface and the inactive side groups remain in the interior.
Solution 3
(a)
|
Molecule |
Structure |
|
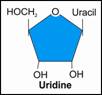
i. Uridine |
|
|
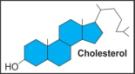
ii. Cholesterol |
|
|
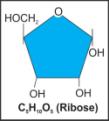
iii. Ribose |
|
|
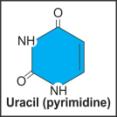
iv. Uracil |
|
|
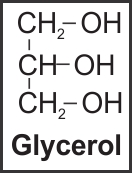
v. Glycerol |
|
|
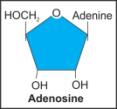
vi. Adenosine |
|
|
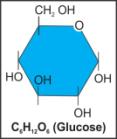
vii. Glucose |
|
|
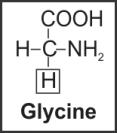
viii. Glycine |
|
|
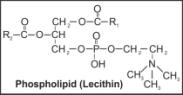
ix. Phospholipid |
|
|
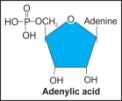
x. Adenylic acid |
|
(b)
|
|
Compound |
Manufacturer |
Buyer |
|
i. |
Starch products |
Kosha Impex (P) Ltd. |
Research laboratories, educational institutes and other industries which use biomolecules as a precursor for making other products. |
|
ii. |
Liquid glucose |
Marudhar apparels |
|
|
iii. |
Various enzymes such as amylase, protease and cellulase |
Map (India) Ltd. |
Solution 4
List of proteins used as therapeutic agents: Insulin, Oxytocin, Antidiuretic Hormone (ADH), Thrombin, Fibrinogen, Renin, Immunoglobulin, Diastase and Streptokinase.
Other applications:
i. Cosmetics: Proteins are used in beauty creams and shampoos. Example: Casein
ii. Sweeteners: Some proteins are used as sweeteners. Example: Thaumatin is a low-calorie sweetener and flavour modifier.
iii. Dietary proteins: Proteins are added to dietary supplements for body building and maintenance of health.
Solution 5
Triglycerides are formed from a single molecule of glycerol combined with three fatty acids on each of the OH groups through ester bonds.
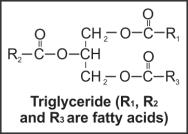
In pure fat, all the three fatty acids of a triglyceride are similar (e.g. tripalmitin), while in mixed fat, they are dissimilar (e.g. palmito-oleo-stearin).
Solution 6
Yes. The biomolecules can be represented by the ball and stick model. The bonds which hold the atoms are represented by sticks, whereas the atoms are represented by balls.
Example: In the model of D-glucose, the oxygen atoms are represented by pink balls, the hydrogen atoms by green balls, while the carbon atoms are represented by grey balls.
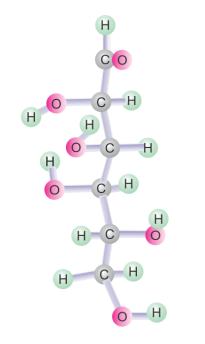
Solution 7
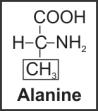
Solution 8
Gums are natural heteropolysaccharides and are formed of a large number of different monosaccharide units linked together by glycosidic bonds. Fevicol is different from gums as it comprises synthetic polymers.
Solution 9
Qualitative tests for proteins, amino acids and fats:
i. Biuret test: The Biuret test for protein identifies the presence of protein by producing light blue to purple colour of the solution.
ii. Grease test for oil: Certain oils give a translucent stain on brown paper. This test can be used to show the presence of fat in vegetable oils.
iii. Ninhydrin test: If Ninhydrin reagent is added to the solution, then the colourless solution changes to pink, blue or purple colour depending on the type of amino acid.
|
Item |
Name of the test |
Procedure |
Result |
Inference |
|
|
i. |
Fruit juice |
Biuret test |
Fruit juice + Biuret's reagent |
Colour changes from light blue to purple. |
Presence of protein. |
|
|
|
Grease test |
Add a few drops of fruit juice on brown paper. |
No translucent drop formed. |
Absence of fats and oils. |
|
|
|
Ninhydrin test |
Fruit juice + Ninhydrin reagent + Boil for 5 min |
Colourless solution changes to pink, blue or purple colour. |
Presence of amino acids. |
|
ii. |
Saliva |
Biuret test |
Saliva + Biuret's reagent |
Colour changes from light blue to purple. |
Presence of protein. |
|
|
|
Grease test |
Add a few drops of saliva on brown paper. |
No translucent drop formed. |
Absence of fats and oils. |
|
|
|
Ninhydrin test |
Saliva + Ninhydrin reagent + Boil for 5 min |
Colourless solution changes to pink, blue or purple colour. |
Presence of amino acids. |
|
iii. |
Sweat |
Biuret test |
Sweat + Biuret's reagent |
No colour change |
Absence of proteins. |
|
|
|
Grease test |
Add a few drops of sweat on brown paper. |
Oily appearance |
Fats/oils may be present. |
|
|
|
Ninhydrin test |
Sweat + Ninhydrin reagent + Boil for 5 min |
No colour change; solution remains colourless. |
Absence of amino acids. |
|
iv. |
Urine |
Biuret test |
Urine + Biuret's reagent |
Colour changes from light blue to purple. |
Presence of protein. |
|
|
|
Grease test |
Add a few drops of urine on brown paper. |
Little bit of oily appearance. |
Fats may or may not be present. |
|
|
|
Ninhydrin test |
Urine + Ninhydrin reagent + Boil for 5 min |
Colourless solution changes to pink, blue or purple colour. |
Presence of amino acids. |
Solution 10
About 100 billion tonne of cellulose is formed annually in the biosphere out of 170 billion tonne of total organic matter. Paper making consumes about 0.5 billion tonne of wood. Trees are also used to fulfil other requirements of human beings such as medicines, timber and food. A rough estimate shows that food grains constitute 1.5 billion tonne. Full wood required is 2 billion tonne. Hence, it is difficult to calculate the annual consumption of plant material by man. The increase in consumption of cellulose has resulted in a great loss of vegetation.
Solution 11
Properties of enzymes:
i. Chemical nature: Enzymes are generally complex macromolecules of globular proteins. They do not initiate a chemical reaction but increase the rate of chemical reaction.
ii. Molecular weight: Being proteinaceous in nature, the enzymes are giant molecules with a molecular weight of 6000 to 4,600,000.
iii. Changeless form: Enzymes are not transformed in the chemical reaction. They combine temporarily with the substrate molecules but are not consumed or changed permanently in the reaction they catalyse.
iv. Action specificity: The enzymes are specific in their action. They catalyse only a particular kind of reaction and may even react on a particular substrate. Example: Enzyme maltase acts on sugar maltose but not on lactose or sucrose.
v. Heat sensitivity: All enzymes are heat sensitive, i.e. they are thermolabile.
vi. pH sensitivity: Each enzyme functions at a particular pH. Example: Pepsin, a digestive enzyme in the stomach, works best at a pH 2. A change in the pH makes the enzyme ineffective.