Class 9 FRANK Solutions Chemistry Chapter 4 - The Language of Chemistry
56
The Language of Chemistry Exercise 56
Solution 1
Symbol - It is the short form or abbreviation used for the name of an element. It represents one atom of that element.
Formula - Formula of a compound represents the composition of a molecule of the substance in terms of the symbols of the elements present in the molecule.
Formula - Formula of a compound represents the composition of a molecule of the substance in terms of the symbols of the elements present in the molecule.
Solution 2
(a) CaCO3
(b) MgSO4
(c) Fe2 (SO4)3
(d) CaHCO3
(e) CuI
(f) K2Cr2O7
(g) KMnO4
(h) Na2SO4
(i) Mg (NO3)2
(j) Ca3 (PO4)2
(b) MgSO4
(c) Fe2 (SO4)3
(d) CaHCO3
(e) CuI
(f) K2Cr2O7
(g) KMnO4
(h) Na2SO4
(i) Mg (NO3)2
(j) Ca3 (PO4)2
Solution 3
(a) Valency - The combining capacity of an element is called its valency.
(b) Helium < Sodium < Magnesium < Carbon < Phosphorous
(b) Helium < Sodium < Magnesium < Carbon < Phosphorous
Solution 4
Law of conservation of matter governs a completely balanced equation. It states that "matter can neither be created nor destroyed."
Solution 5
A symbol signifies one atom of that element.
Solution 6
Latin names of the following compounds are-
Iron - Ferrum
Tin - Stannum
Lead - Plumbum
Sodium - Natrium
Potassium - Kalium
Mercury - Hydragyrum
Iron - Ferrum
Tin - Stannum
Lead - Plumbum
Sodium - Natrium
Potassium - Kalium
Mercury - Hydragyrum
Solution 7
The equation in which the total number of atoms of each element in the reactants, on the left side of the equation is same as the number of atoms in the products formed, on the right side of the equation is called as balanced chemical equation.
Solution 8
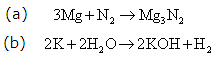
Solution 9
A chemical equation gives information about-
(i) What substances enter into a given reaction (reactants) and what products are formed as a result of the reaction.
(ii) The quantities of the reactants and the product formed.
(iii) The optimum conditions of temperature and pressure.
(i) What substances enter into a given reaction (reactants) and what products are formed as a result of the reaction.
(ii) The quantities of the reactants and the product formed.
(iii) The optimum conditions of temperature and pressure.
Solution 10
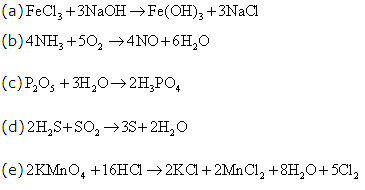
Solution 11
H2 means hydrogen, O4 means oxygen and S means sulphur in the formula of H2SO4.
Solution 12
(a) The highest valency of the element Z is six.
(b) The formula of the fluoride of Z will be ZF6.
(b) The formula of the fluoride of Z will be ZF6.
Solution 13
The three valencies of element are-
(i) Two-Since the element X combines with two hydrogen to form H2X and two atoms of X combines with one carbon to form CX2.
(ii) Four-Since the element X combines with two oxygen to form XO2.
(iii) Six-Since the element X combines with three oxygen to form XO3.
(i) Two-Since the element X combines with two hydrogen to form H2X and two atoms of X combines with one carbon to form CX2.
(ii) Four-Since the element X combines with two oxygen to form XO2.
(iii) Six-Since the element X combines with three oxygen to form XO3.
Solution 14
Variable valency - Some elements are capable of showing more than one valency in their compounds called variable valency.
Some elements show variable valency i.e. more than one valency since these elements have more than one common valency state.
Some elements show variable valency i.e. more than one valency since these elements have more than one common valency state.
Solution 15
Chemical formula - It represents the composition of a molecule of the substance in terms of the symbols of the elements present in the molecule. The rule for writing the formula is criss-cross method.
(i) The positive and negative radicals are represented by their symbols and written side by side with the correct valency written below each.
(ii) The valencies are divided by their highest common factor if any to get the simplest ratio.
(iii) These numbers are then interchanged and written as subscripts.
(i) The positive and negative radicals are represented by their symbols and written side by side with the correct valency written below each.
(ii) The valencies are divided by their highest common factor if any to get the simplest ratio.
(iii) These numbers are then interchanged and written as subscripts.
Solution 16
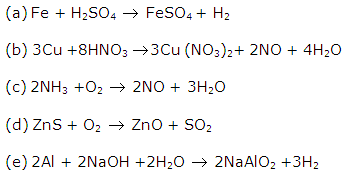
Solution 17
(a) Sodium hydrogencarbonate
(b) Sodium hexacyanoferrate(III)
(c) Manganese(II) borate
(d) Calcium phosphate
(e) Potassium manganate(VI)
(b) Sodium hexacyanoferrate(III)
(c) Manganese(II) borate
(d) Calcium phosphate
(e) Potassium manganate(VI)
Solution 18
Co stands for cobalt which is an element while CO stands for carbon monoxide which is a compound.
Solution 19
Radical-
A radical is an atom or a group of atoms of same or different elements that behaves in the manner of positive or negative ion. Radicals have their own combining power(valency) and chemical formulae.
Examples-
Monovalent radicals -H-,OH-,Cl-,NO3-,H+,Na+,K+,NH4+
Trivalent radicals-PO43- ,Fe(CN)63-,AsO3 3-,N3-,Fe3+,Al3+,Bi3+,Au3+
A radical is an atom or a group of atoms of same or different elements that behaves in the manner of positive or negative ion. Radicals have their own combining power(valency) and chemical formulae.
Examples-
Monovalent radicals -H-,OH-,Cl-,NO3-,H+,Na+,K+,NH4+
Trivalent radicals-PO43- ,Fe(CN)63-,AsO3 3-,N3-,Fe3+,Al3+,Bi3+,Au3+
Solution 20
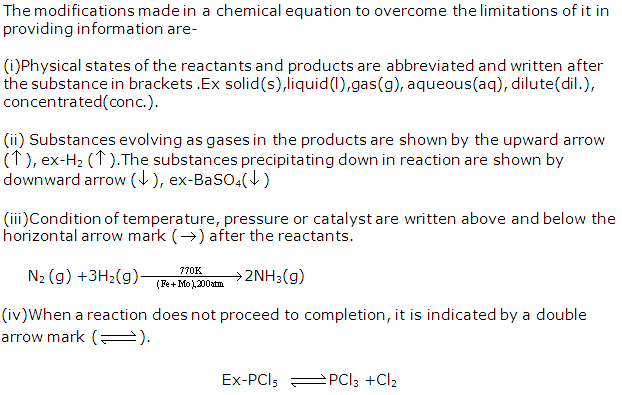
Solution 21
(a) Anion - Negatively charged radicals are termed as anions.
(b) Cation - Positively charged radicals are termed as cations.
(b) Cation - Positively charged radicals are termed as cations.
Solution 22
Disadvantages associated with hit and trial method of balancing of equations-
(i) It is tedious and takes a long time.
(ii) The method is rather difficult for balancing such equations which contain the same element being repeated in a number of compounds.
(iii) It does not give any information regarding the mechanism of the reaction.
(i) It is tedious and takes a long time.
(ii) The method is rather difficult for balancing such equations which contain the same element being repeated in a number of compounds.
(iii) It does not give any information regarding the mechanism of the reaction.

