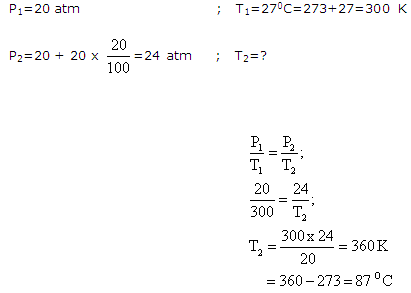Class 9 FRANK Solutions Chemistry Chapter 2: Study of Gas Laws
Gain conceptual knowledge with Frank Solutions for ICSE Class 9 Chemistry Chapter 2 Study of Gas Laws. Study Boyle’s Law and Charles’ Law by practising our textbook solutions. Also, go through questions and answers on standard temperature and pressure.
Practise problems on calculating the final volume of gas, the volume of dry air, and more. Study gas laws for your exam thoroughly with the ICSE Class 9 Chemistry Frank solutions and Selina solutions at TopperLearning. Also, browse through our learning materials such as sample paper solutions, video lessons, online practice tests and more to help you understand concepts better.
21
22
Study of Gas Laws Exercise 21
Solution 1
An ideal gas can be characterized by three state variables:
(i) Absolute pressure (P),
(ii) Volume (V), and
(iii) Absolute temperature (T).
(i) Absolute pressure (P),
(ii) Volume (V), and
(iii) Absolute temperature (T).
Solution 2

Solution 3
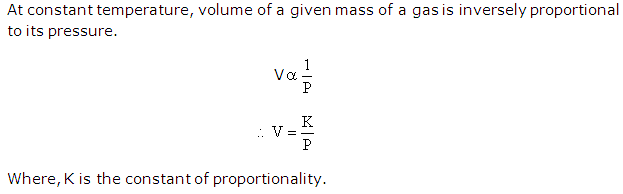
Solution 4
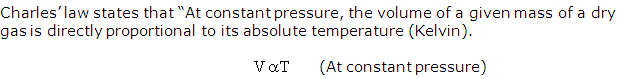
Solution 5
Kelvin zero is -273.15oC.
Solution 6

Solution 7
The standard temperature and pressure (STP) by general convention are 0OC(273 K) and 1 atm(760 mm Hg).
Solution 8
(a) The value of standard temperature is (i) 0oC and (ii) 273 K
(b)The value of standard pressure is (i) 1 atm, (ii) 760 mm of Hg, (iii)76 cm of Hg, (iv)760 torr
(b)The value of standard pressure is (i) 1 atm, (ii) 760 mm of Hg, (iii)76 cm of Hg, (iv)760 torr
Solution 9
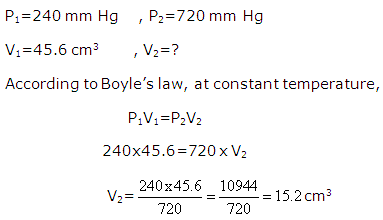
Solution 10
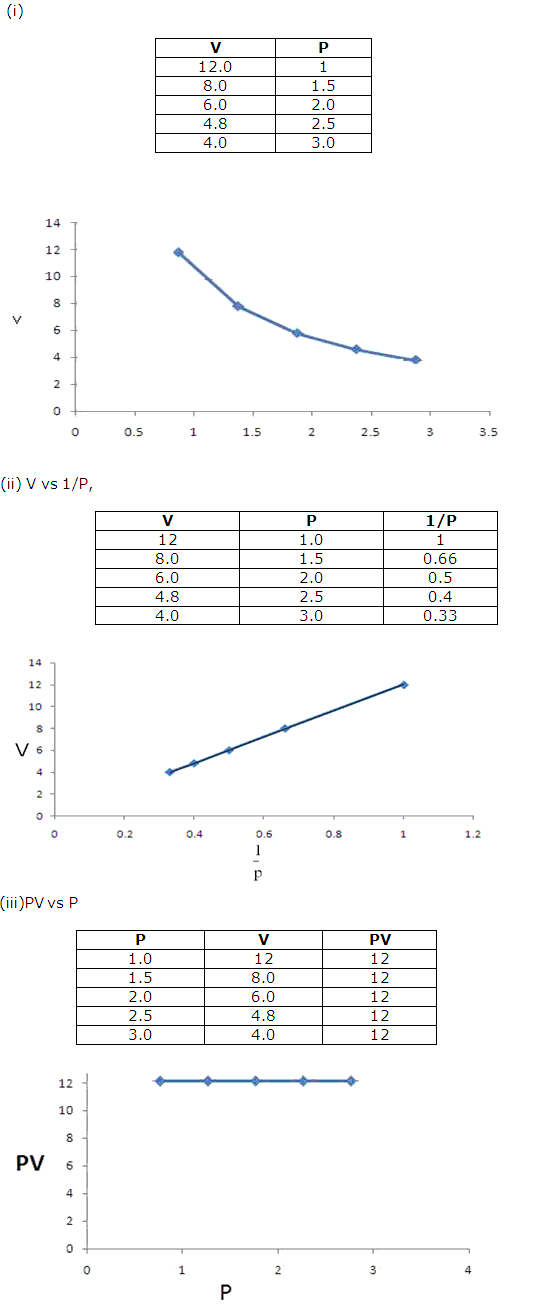
Solution 11
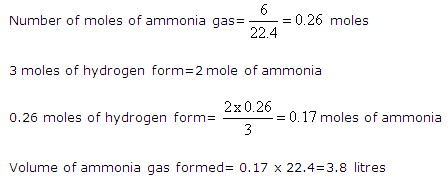
Solution 12
There is simultaneous effect of temperature and pressure changes on the volume of a given mass of a gas. So, when stating the volume of a gas, the pressure and temperature should also be given.
Study of Gas Laws Exercise 22
Solution 13
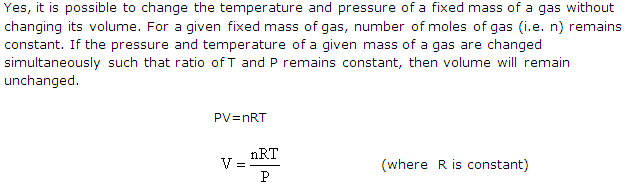
Solution 14
(a) Volume of a gas would be reduced to zero at 0 K (-2730C).All temperatures on the Kelvin scale are positive, so Kelvin scale has been adopted for chemical calculation.
(b) At absolute zero temperature, volume of a gas would be reduced to zero. Theoretically,this is the lowest temperature that can be reached. At this temperature all molecular motions cease. Thus, practically this temperature is impossible to attain because on cooling gases liquefy and Charles' law is no more applicable.
(c) According to combined gas law equation, there is simultaneous effect of temperature and pressure changes on the volume of a given mass of a gas. So, when stating the volume of a gas, the pressure and temperature should also be given.
(b) At absolute zero temperature, volume of a gas would be reduced to zero. Theoretically,this is the lowest temperature that can be reached. At this temperature all molecular motions cease. Thus, practically this temperature is impossible to attain because on cooling gases liquefy and Charles' law is no more applicable.
(c) According to combined gas law equation, there is simultaneous effect of temperature and pressure changes on the volume of a given mass of a gas. So, when stating the volume of a gas, the pressure and temperature should also be given.
Solution 15

Solution 16
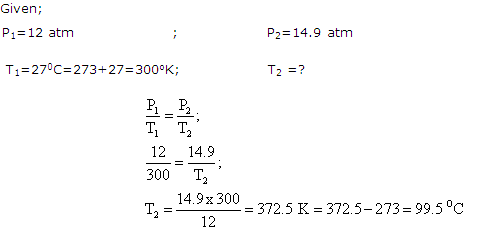
Solution 17
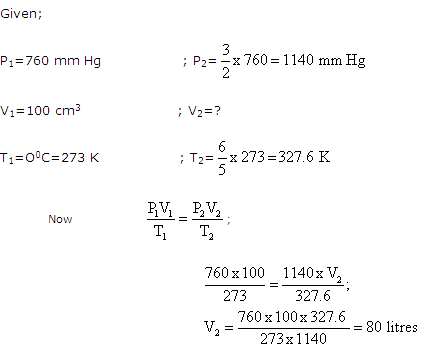
Solution 18
(a) True
(b) False
(c) False
(d) False
(e) False
(b) False
(c) False
(d) False
(e) False
Solution 19
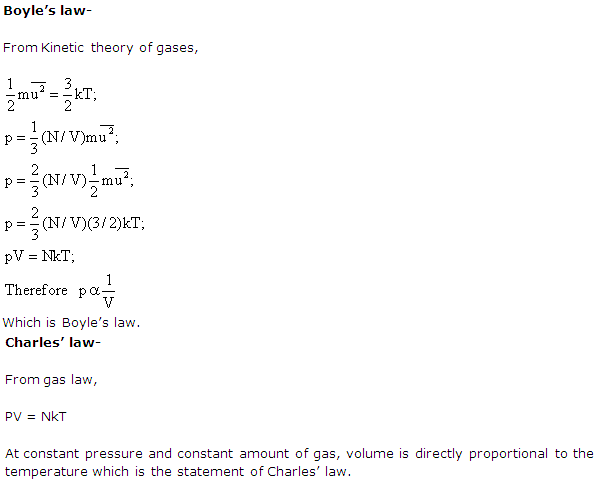
Solution 20
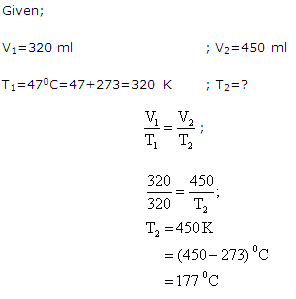
Solution 21
We trap a definite quantity of air in the closed vessel. At any point, the pressure on the air is equal to the atmospheric pressure plus the pressure due to the excess mercury column in the open end tube. By pouring mercury in the tube, we increase the pressure on the air and measure its volume under that pressure. We thus obtain a set of data for the volume of a fixed mass of air under different pressures.
For a given mass of air at constant temperature, the following observations are made-
(i) The volume of air decreases with increasing pressure and vice versa.
(ii) The proportion by which the volume decreases or increases is the same by which the pressure increases or decreases.
For a given mass of air at constant temperature, the following observations are made-
(i) The volume of air decreases with increasing pressure and vice versa.
(ii) The proportion by which the volume decreases or increases is the same by which the pressure increases or decreases.
Solution 22
(a) Pressure will also be doubled.
(b) Pressure will be double.
(b) Pressure will be double.
Solution 23
(a) 273
(b) absolute zero
(c) absolute temperature
(d) the average kinetic energy
(b) absolute zero
(c) absolute temperature
(d) the average kinetic energy
Solution 24
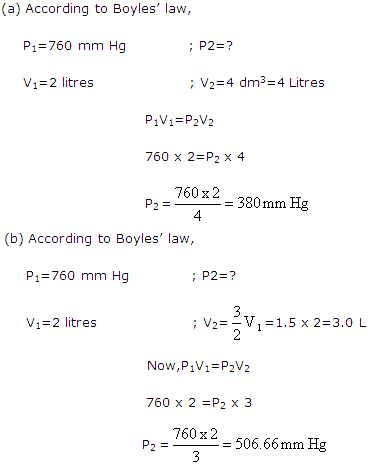
Solution 25
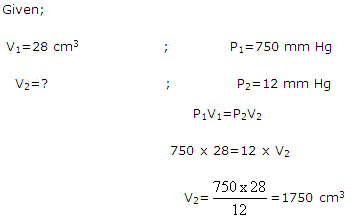
Solution 26
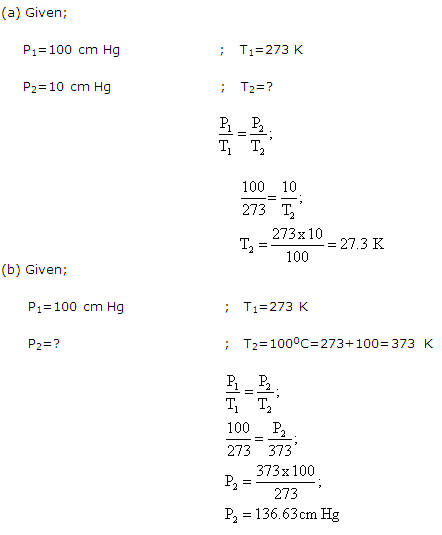
Solution 27
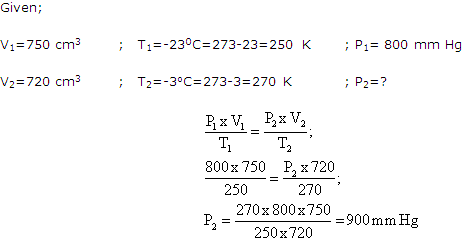
Solution 28
As weather balloon go higher into the atmosphere, the air becomes less dense, so air pressure drops. Because of this, the air that is already inside the balloon expands to cope with the difference in pressure. The end result is that the balloon expands making it larger.
Solution 29