Class 9 FRANK Solutions Chemistry Chapter 7: Atomic Structure
113
114
Atomic Structure Exercise 113
Solution 1
Solution 2
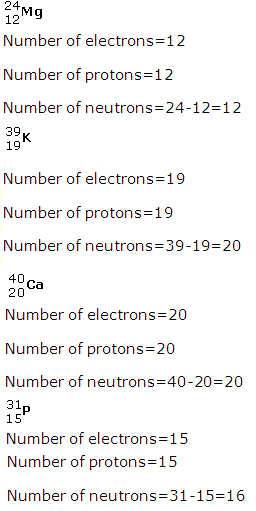
Solution 3
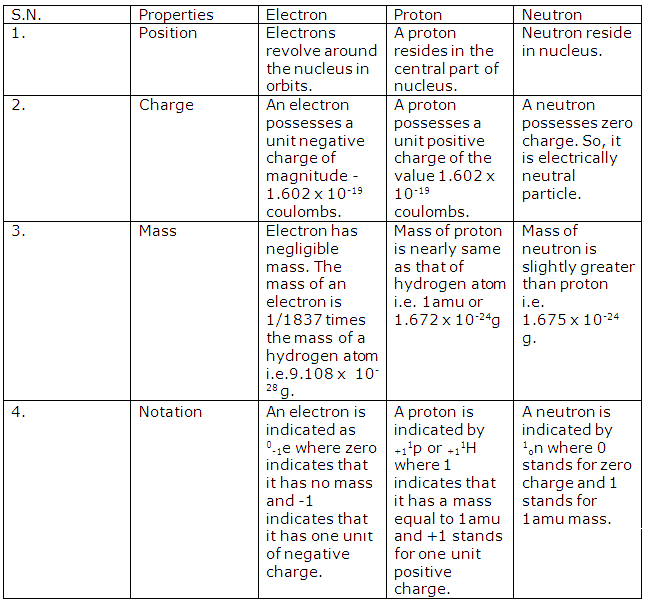
(a) Mass of an atom - Protons and neutrons
(b) Size of an atom - Electrons
(b) Size of an atom - Electrons
Solution 4
Three fundamental particles of an atom are-
(a) Proton
(b) Electron
(c) Neutron
(a) Proton
(b) Electron
(c) Neutron
Solution 5
(a) An atom - An atom is the smallest particle of an element which may or may not be capable of independent existence.
(b) An element - An element is usually defined as a pure substance that contains only one kind of particles. These particles may be atoms or molecules.
(b) An element - An element is usually defined as a pure substance that contains only one kind of particles. These particles may be atoms or molecules.
Solution 6
Atomic number - The number of protons present in the nucleus of an atom is called the atomic number of the atom.
It is denoted by 'Z'.
It is denoted by 'Z'.
Solution 7
The protons and neutrons are collectively called as nucleons.
Solution 8
Isotopes are atoms of the same element, having the same atomic number, same chemical properties but different mass number, i.e., the atoms differ in the number of neutrons. The three isotopes of hydrogen atoms are-
(i) Protium
(ii) Deuterium
(iii) Tritium
(i) Protium
(ii) Deuterium
(iii) Tritium
Solution 9
Electrons take part in a chemical reaction. Therefore, the chemical properties of an element depend upon the electronic configuration. Since, isotopes of an element have the same atomic number and hence same electronic configuration. So, they exhibit the same chemical properties.
Solution 10
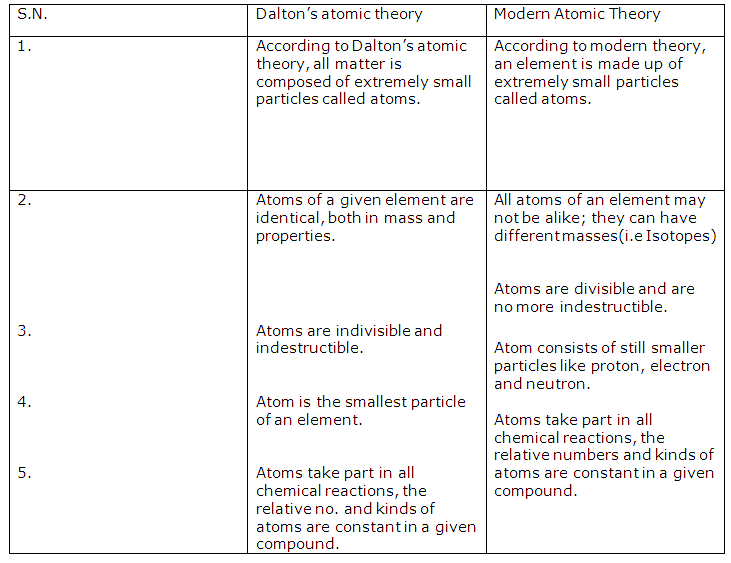
Solution 11
The outermost orbit of an element is called valence shell. The electrons present in the outermost orbit (valence shell) of an element are called valence electrons.
Solution 12
Atoms may have incomplete octet. During the formation of a molecule, an atom of a particular element gains, loses or shares electrons until it acquires a stable configuration of eight electrons in its valence shell.
Solution 13
(a) Atomic number = Number of protons = 20
(b) Mass number = Number of protons +Number of electrons = 20 + 20 = 40
(c) Electronic configuration = 2, 8, 8, 2
(d) Valency = 2
(b) Mass number = Number of protons +Number of electrons = 20 + 20 = 40
(c) Electronic configuration = 2, 8, 8, 2
(d) Valency = 2
Solution 14
(a) A=1
B=3
C=2
D=1
E=1
(b) E contains the greatest number of neutrons.
(c) A contains the least number of electrons.
(d) B contains equal number of electrons and neutrons.
(e) A is a metal.
(f) Fluorine is the most reactive of the non-metals.
(g) Both are electronegative and non-metals.
(h) D and E belongs to a particular family.
(i) A3B will be the formula if atoms of A combines with atoms of B.
(j) 'B' will combine in a trivalent element while 'C' will combine as a bivalent element.
B=3
C=2
D=1
E=1
(b) E contains the greatest number of neutrons.
(c) A contains the least number of electrons.
(d) B contains equal number of electrons and neutrons.
(e) A is a metal.
(f) Fluorine is the most reactive of the non-metals.
(g) Both are electronegative and non-metals.
(h) D and E belongs to a particular family.
(i) A3B will be the formula if atoms of A combines with atoms of B.
(j) 'B' will combine in a trivalent element while 'C' will combine as a bivalent element.
Atomic Structure Exercise 114
Solution 15
"During the formation of the molecule, an atom of a particular element gains, loses electrons or shares electrons until it acquires a stable configuration of eight electrons in its valence shell" i.e. until it acquires octet.
Solution 16
K can accommodate maximum of 2 electrons.
L can accommodate maximum of 8 electrons.
M can accommodate maximum of 18 electrons.
L can accommodate maximum of 8 electrons.
M can accommodate maximum of 18 electrons.
Solution 17
Cathode rays are formed at the negative electrode of the discharge tube experiment.
Solution 18
(i) In K maximum number of 2 electrons can be accommodated.
(ii) In L maximum number of 8 electrons can be accommodated.
(iii) In M maximum number of 18 electrons can be accommodated.
(iv) In N maximum number of 32 electrons can be accommodated.
(ii) In L maximum number of 8 electrons can be accommodated.
(iii) In M maximum number of 18 electrons can be accommodated.
(iv) In N maximum number of 32 electrons can be accommodated.
Solution 19

Solution 20
Electronic configuration of magnesium is -2, 8, 2. Since, it has 2 electrons in its valence shell, so its valency is 2.
Solution 21
Number of electrons in Sodium = 11
Number of protons in sodium = 11
Number of neutrons in sodium = 12
Number of nucleons in sodium = 23
Number of protons in sodium = 11
Number of neutrons in sodium = 12
Number of nucleons in sodium = 23
Solution 22
Inert elements are the elements which have completely filled valence shell. Since, they are already stable and do not need more electrons, they do not combine with other atoms. So, they exist as monoatoms in molecule.
Solution 23
(a) Hydrogen
(b) K shell.
(c) Magnesium
(d) Isotopes
(e) Helium has zero valency.
(b) K shell.
(c) Magnesium
(d) Isotopes
(e) Helium has zero valency.
Solution 24
(a) The number of protons = 9
(b) The number of neutrons = 19 - 9 = 10
(c) The number of electrons = 9
(b) The number of neutrons = 19 - 9 = 10
(c) The number of electrons = 9
Solution 25
Atomic number is the number of protons of an atom which is unique to an atom but mass number is the total of number of protons and number of neutrons which may or may not be same to other atoms since there is probability of combination of number of protons and number of neutrons be same for two atoms.
