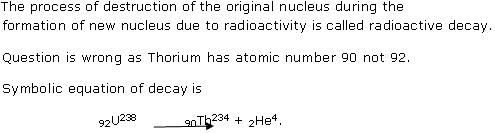Class 10 FRANK Solutions Physics Chapter 6 - Modern Physics - Exercises and MCQ
Get Physics learning support with our Frank Solutions for ICSE Class 10 Physics Chapter 6 Modern Physics Exercises and MCQ. Through our Frank Solutions, relearn Physics concepts such as isotopes, radioactive decay, radioactivity, gamma radiations etc. Understand the uses of radioactive isotopes in agriculture, industries and in the medical field.
At the TopperLearning study portal, we also give you seamless access to ICSE Class 10 Physics online learning materials. Our resources like practice tests, concept videos, question papers and more will complement your efforts to clear your Physics exam with a top score.
Modern Physics - Exercises and MCQ Exercise 266
Solution 1
(a) Mass number of copper = 63
Atomic number of copper = 29
As atomic number of element gives number of proton and electrons while mass number of element gives number of protons +number of neutrons.
So, number of protons in copper = atomic number of copper = 29.
Number of electron in copper = number of protons in copper =29.
Number of neutrons = mass number atomic number = 63 29 =34.
So answer is (iii) as copper contains 29 protons and 29 electrons.
Modern Physics - Exercises and MCQ Exercise 267
Solution 2

Solution 3
(b) Neutrons have (iv) no electric charge.
Solution 4
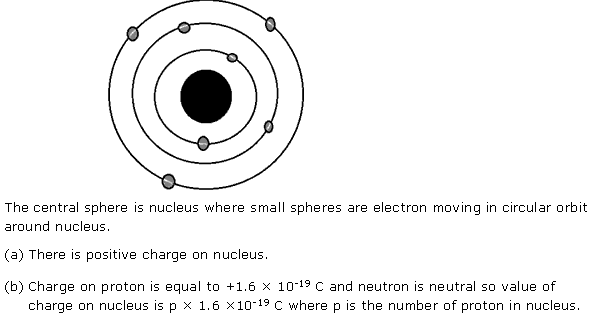
Solution 5

Solution 6
Atomic number of uranium = 92
As atomic number of element gives number of proton and electrons while mass number of element gives number of protons +number of neutrons.
So, number of protons in uranium = atomic number of uranium = 92.
(b) Number of electron in uranium = number of protons in uranium =92.
(c)The atoms of same elements having the same atomic number Z but different mass number A are called isotopes. So, for another isotope of uranium mass number 235 changes.
(d)Number of protons in isotopes is same and as U238 is isotope of 92U235. So, number of protons in U235 is also 92.
Solution 7

Solution 8
Solution 9

Solution 10
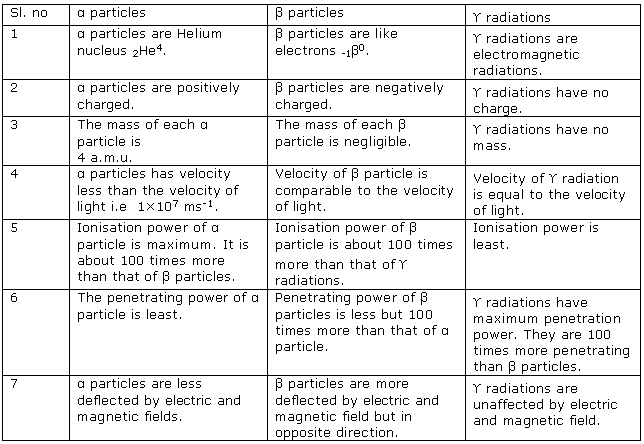
Solution 11
Solution 12
Solution 13
Following precautions should be taken while handling the radioactive substances.
(i) The sources should only be handled by the forceps provided and never touched by hand.
(ii) They should never be pointed towards a person.
(iii) Food should not be taken where the sources are being used, as it may be contaminated.
(iv) Never smoke near a radioactive source.
Solution 14

Solution 15
Sources of background radiations are:
(a) Radiation from the sun.
(b) Rocks in the earth which contain traces of radioactive substances.
(c) Naturally occurring isotopes.
(d) Artificial radioisotopes.
No, it is not possible to keep ourselves away from these radiations.
Solution 16
In controlled fission reaction, out of 3 neutrons released in nuclear fission, 2 neutrons are absorbed and one neutron is available to cause fission so that nuclear reaction takes place at a steady rate and slows down.
Solution 17
|
Nuclear Fission |
Nuclear fusion |
|
In fission reactions, a heavy nucleus is split into two nuclei with smaller mass numbers. |
In fusion reactions, two light nuclei are combined to form a heavier, more stable nucleus. |
|
The amount of energy required to split two atoms in a fission reaction is less as compared to fusion reaction. |
A tremendous large amount of energy is required to bring two or more protons close enough to overcome their electrostatic force of repulsion. |