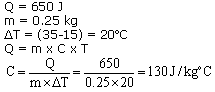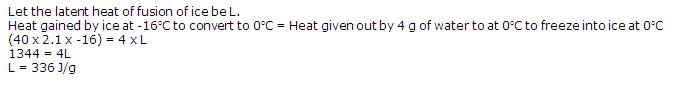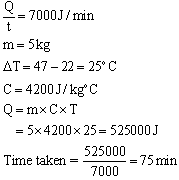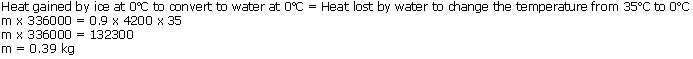Class 10 FRANK Solutions Physics Chapter 5 - Heat - Exercises and MCQ
Understand heat-related concepts with Frank Solutions for ICSE Class 10 Physics Chapter 5 Heat Exercises and MCQ. Practise Physics numericals based on heat by learning the problem-solving techniques used in our step-wise solutions. Also, practise MCQs with our Frank Solutions.
Why is a steam burn considered worse than a hot water burn? TopperLearning’s Physics experts will guide you with correct scientific reasons for such scenarios. To learn more about heat concepts, utilise our ICSE Class 10 Physics concept videos, practice tests and revision notes.
Heat - Exercises and MCQ Exercise 245
Solution 1
(b)2000 / (4 x 3) J/kg oC
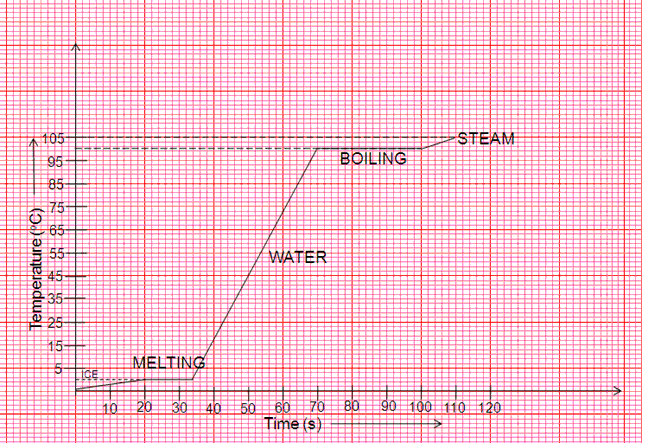
(c)AB and CD
(d)Latent heat is emitted
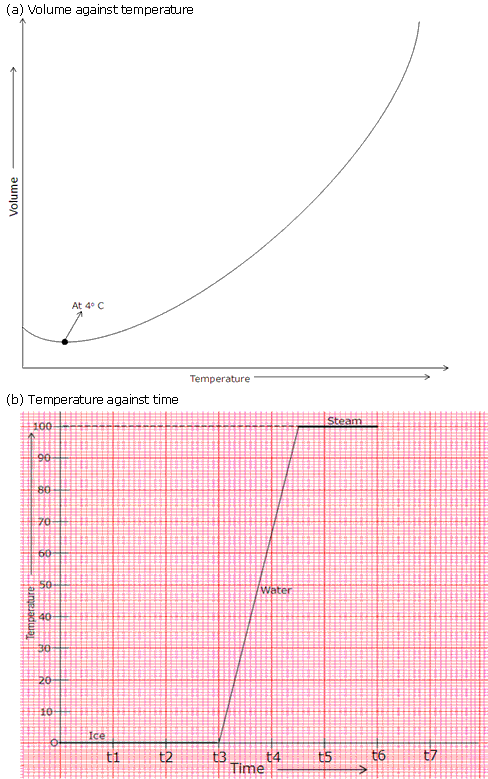
(e)The first time when temperature is constant represents change of state from solid to liquid and the second time temperature is constant represents change of state from liquid to vapour.
Heat - Exercises and MCQ Exercise 246
Solution 2
(a)Heat capacity of a body is the quantity of heat required to raise its temperature by 1oC. It depends upon the mass and the nature of the body.
Units: J/oC or calorie/oC
(b)Change in temperature = (50-30) = 20oC
Amount of heat required, Q = m x C x ?T
= 0.5 x 4200 x 20 = 42000 J
Solution 3
(a)This means that 390 J of heat is required to raise the temperature of 1kg of copper by 1oC.
(b)Change in temperature = (100-30) = 70oC = 70 K
Amount of heat given out, Q = m x C x T
= 0.6 x 900 x 70 = 37800 J
Solution 4
Solution 5
Solution 6
Solution 7
Solution 8
Solution 9
Solution 10
Solution 11
Solution 12
Solution 13
Solution 14

Heat - Exercises and MCQ Exercise 247
Solution 15
Solution 16
Solution 17
Solution 18
(a) Ice melts under pressure. So, when the steel blades of the skates pressed on the ice, the ice melts. The water formed makes the skates slide easily over the ice, reducing friction. So, when we are skating on ice, we are skating on a thin film of water, which acts like lubricating oil. Nothing such happens in case of glass.
(b) Sand improves the friction between car tyres and the road, so cars don't skid on icy surfaces. Salt is spread so as to decrease the melting point of ice. Ice on the roads melt, making the roads less slippery.
(c) Steam burn is worse than a hot water burn because 1 g of steam gives out 540 calories of additional heat.
(d) Lumps of ice cool better than cold water because each gram of ice requires additional 80 calories of heat to get converted into water. Hence, cooling capacity of lumps of ice is more than cold water.
Solution 19
Solution 20
Solution 21
Solution 22
Solution 23
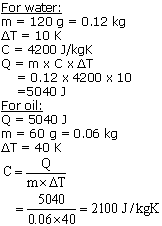
Solution 24
Solution 25
Solution 26
Solution 27
Solution 28
Solution 29
Solution 30
Solution 31
Heat - Exercises and MCQ Exercise 248
Solution 32
Steam at 100oC will produce more severe burns because every gram of steam gives out 2260 J of heat energy while condensing. This much amount of heat is additional to the heat contained in one gram of boiling water.
Solution 33
Ice cream appears colder to mouth than water at 0oC because it can extract approximately 80 cal/g (latent heat of fusion of ice)more heat from as compared to water at 0 oC.
Solution 34
Solution 35
Solution 36
Solution 37
1 gram of ice at 0oC requires 80 calories of heat to get converted into 1 gram of water at 0oC. So, water has more heat.
Solution 38
1 gram of water at 100oC requires 540 calories of heat to get converted into 1 gram of steam at 100oC. So, steam has more heat.
Solution 39
1 gram of ice at 0oC requires additional 80 calories of heat to get converted into water at 0oC. Then, heat is provided to raise the temperature to 10oC. Therefore, ice requires more heat than water and the additional heat is known as 'Latent heat of fusion of ice'.