Change of State and Latent Heat Exercise 241
Solution 1
Specific heat is the amount of heat required to raise the temperature of 1 kg of the substance through 1oC.
It is not same as heat capacity.
Solution 2
SI unit of specific heat is J kg-1K-1.
Solution 3
Specific Heat.
Solution 4
Principle of Calorimetry:
When a hot body is mixed or kept in contact with a cold body, there is a transfer of heat from hot body to cold body such that
Total heat gained by colder body = Total heat lost by the hot body,
if there is no loss of heat to the surroundings.
Solution 5
Thermal capacity of a body is the quantity of heat required to raise its temperature by 1oC.
Solution 6
The product of mass and specific heat is known as heat capacity.
Solution 7
No, specific heat does not depend on temperature. It is constant for a substance.
Solution 8
Copper road becomes warmer than an aluminium rod of the same mass because copper has lower heat capacity than aluminium.
Solution 9
The amount of heat required to raise the temperature of a body by 1oC is called Heat capacity.
Solution 10
Heat capacity has units J/oC.
Solution 11
Specific heat of water is 4200 J kg-1K-1.
Solution 12
The substances like water which have high heat capacity warm up more slowly than substances like iron which have low heat capacity.
Solution 13
Latent heat is the quantity of heat absorbed or released by a substance undergoing a change of state, such as ice changing to water or water to steam, at constant temperature.
Solution 14
SI unit of latent heat is J/kg.
Solution 15
It means that 1 g of ice at 0oC absorbs 336 J of heat energy to convert into water at 0oC.
Solution 16
When a liquid is solidified, it may either expand or contract. As water freezes to form ice it expands and increases in volume by 10 per cent.
Solution 17
The melting point of ice decreases on addition of impurities in it.
Solution 18
The melting point of substances which contract on melting (like ice) decreases by the increase in pressure.
Solution 19
Regelation is the phenomenon of melting under pressure and freezing again when the pressure is reduced.
Solution 20
The amount of heat required to change a liquid at its boiling point to vapour at the same temperature is called its latent heat of vaporization.
Solution 21
The boiling point of a liquid increases with the increase in pressure and decreases with the decrease in pressure.
Solution 22
The latent heat of fusion of ice is the amount of heat energy required to change ice at 0oC into water at the same temperature.
Solution 23
The latent heat of vaporization of steam is the amount of heat energy required to change water at 100oC to steam at the same temperature.
Solution 24
The physical quantity which does not change during change of state is temperature of the body.
Solution 25
1 kg of ice at 0oC absorbs 336000 J of heat energy to convert into water at 0oC. Therefore,1 kg of water at 0oC has 336000 J heat energy more than 1 kg ice at 0oC. So, ice appears colder than water.
Solution 26
Steam causes more severe burns than water at 100oC because every gram of steam gives out 2260 J of heat energy while condensing. This much quantity of heat is additional to the heat contained in 1 g of boiling water.
Solution 27
The unit of heat capacity in CGS system is calorie/oC.
Solution 28
1 cal g-1 oC-1 = 239 J kg-1 oC-1
Solution 29
This means that 0.2 cal g-1 oC-1 of heat is required to raise the temperature of 1g of the body by 1oC.
Solution 30
m = 100 g
C = 0.04 cal g-1 oC-1
Heat capacity = m x C = 4 cal oC-1
Solution 31
Heat capacity = mass x specific heat capacity
Solution 32
No, specific heat does not depend on mass of a substance. It is constant for a substance.
Solution 33
Specific heat of water in SI units is 4200 J kg-1 K-1.
Solution 34
Ammonia has the maximum value of specific heat.
Solution 35
Heat gained or lost by a substance depends on the mass of the substance and the nature of the substance.
Solution 36
Oceans cover more than 70% of Earth's surface, making them the world's largest solar collectors. The sun's heat warms the surface water a lot more than the deep ocean water, and this temperature difference creates heat energy. Thus, oceans are known as storehouse of heat energy. Just a small portion of the heat trapped in the ocean could power the world.
Solution 37
Water has a high specific heat capacity. So, water extracts much heat without much rise in temperature. By allowing water to flow in radiator pipes of the vehicles, heat energy form such parts is removed. Hence, it is used as a cooling agent in the radiators of automobiles.
Change of State and Latent Heat Exercise 242
Solution 38
A change of state is a change in the object from a solid to liquid or from a solid to gas or from liquid to solid. Ice changing into water is an example of a change in state.
Solution 39
Heat energy is absorbed during melting of ice.
Solution 40
Heat energy is released during freezing of ice.
Solution 41
Temperature remains constant when ice melts at 0oC.
Solution 42
The molecules in a solid are held by strong intermolecular bonds. For the solid to melt, these bonds have to be broken. Since energy is needed to break the intermolecular bonds, the thermal energy supplied at the melting point is used to do the work to break the intermolecular bonds between the molecules of the solid. Once the intermolecular bonds are broken, the molecules can then move out of their fixed positions. Hence it can then be said that the solid has melted, which is the change of state from solid to liquid. This explains why temperature remains constant during the melting phases.
Solution 43
Whenever a substance goes through a phase change (like boiling), the energy goes into breaking up the interactions between molecules, and so the temperature stays constant until all the interactions are broken. Once whole of the substance has boiled, then any added heat will act to raise them temperature again.
Solution 44
Atmosphere is usually warm during snowfall because each kilogram of ice on melting absorbs 336000 J of heat from atmosphere.
Solution 45
Latent heat of fusion of ice, L=336 J/g
Mass of ice, m=2g
Amount of heat required convert 2g of ice at 0oC into water at 0oC = m x L
= 2 x 336 = 672 J
Solution 46
Latent heat of vapourisation of water, L=540 cal/g
Mass of water, m=100g
Amount of heat required convert 100g of water at 100oC into steam at 100oC = m x L
= 100 x 540 = 5.4 kJ
Solution 47
Ice melts under pressure. So, when the steel blades of the skates pressed on the ice, the ice melts. The water formed makes the skates slide easily over the ice, reducing friction. So, when we are skating on ice, we are skating on a thin film of water, which acts like lubricating oil. Nothing such happens in case of glass.
Solution 48
Bottled drinks are cooled more effectively when surrounded by lumps of ice than by cold water at 0oC because ice appears colder than water at 0oC.
Solution 49
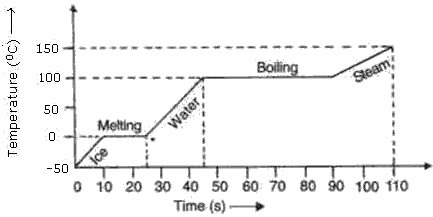
Parts AB and CD correspond to the substance existing in two states.
Solution 50
(e) The first time when temperature is constant represents change of state from solid to liquid and the second time temperature is constant represents change of state from liquid to vapour.
Solution 51
(a)Boiling point of substance is 150oC (because the part BC represents condensation where the vapour changes into liquid without the change in temperature.
(b)DE represents freezing of the substance where the liquid changes into solid at a constant temperature of 100oC.
(c)Melting point is the temperature of the region DE where liquid changes into solid i.e., 100oC.