Class 10 FRANK Solutions Chemistry Chapter 8 - Study of Compounds-I: Hydrogen Chloride
What is aqua regia? What happens when silver nitrate solution is added to dilute HCl? Discover the answers in our Frank Solutions for ICSE Class 10 Chemistry Chapter 8 Study of Compounds-I: Hydrogen Chloride. Also, learn the steps for preparing hydrochloric acid in a laboratory.
With our ICSE Class 10 Chemistry textbook solutions, learn the properties and uses of hydrochloric acid in detail. Revise more to score more. Now, enjoy anytime access to various TopperLearning resources such as concept videos, online tests and more for thorough Chemistry revision.
Study of Compounds-I: Hydrogen Chloride Exercise 198
Solution 1


Study of Compounds-I: Hydrogen Chloride Exercise 199
Solution 2
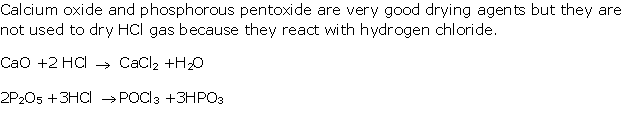
Solution 3
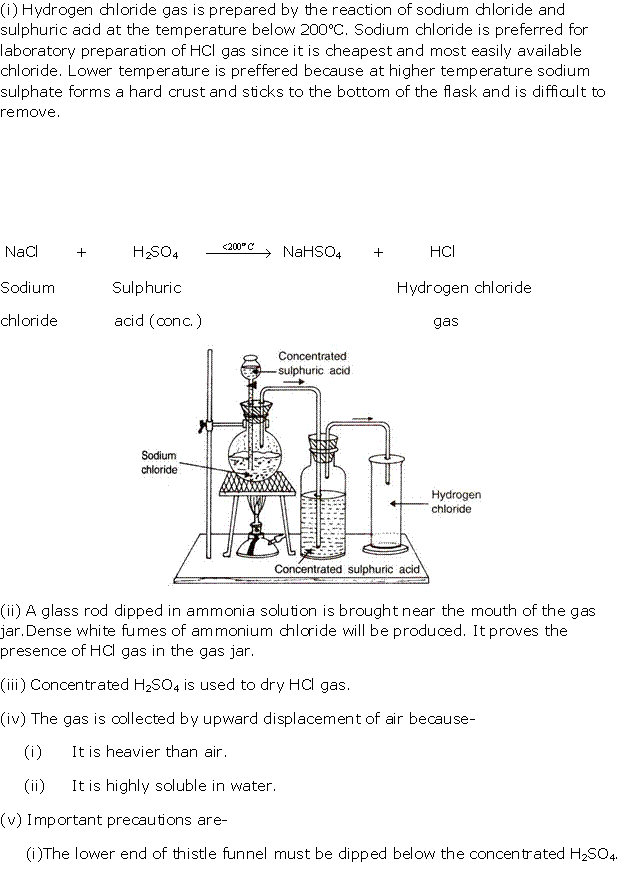

Solution 4
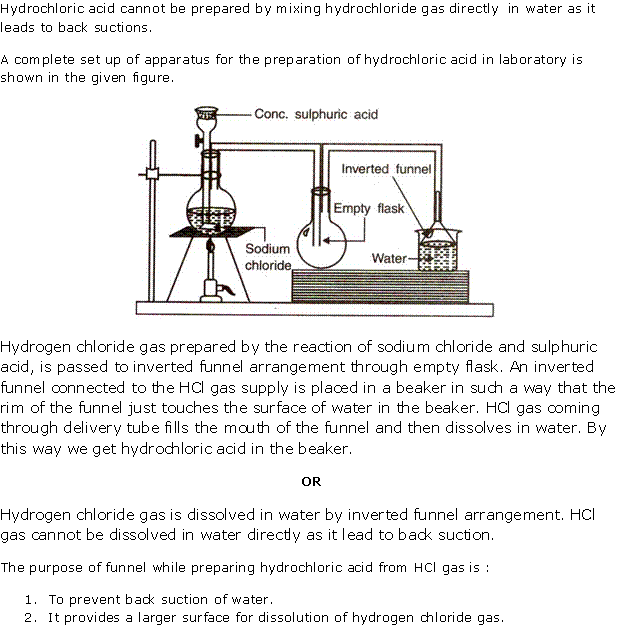
Solution 5
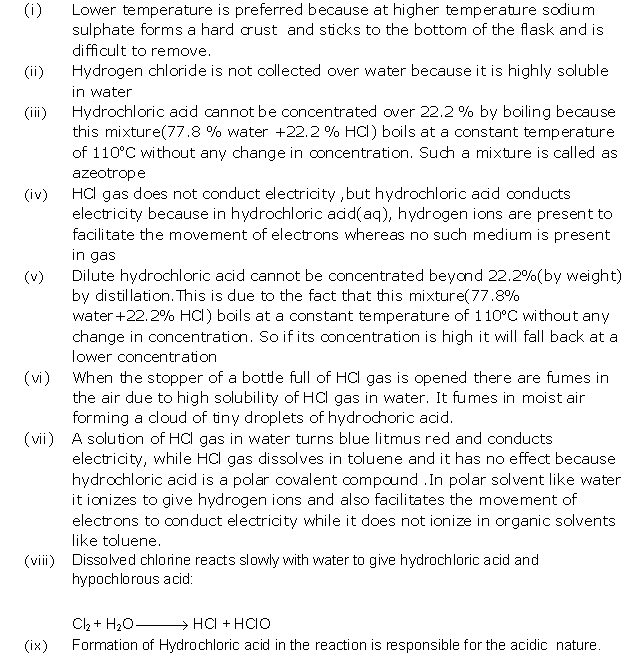
Solution 6

Solution 7

Solution 8
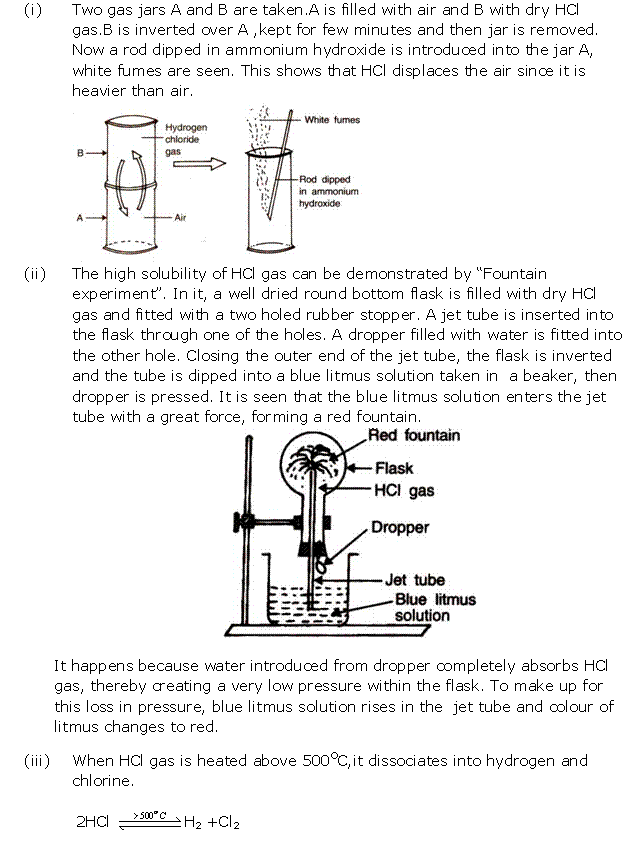
Study of Compounds-I: Hydrogen Chloride Exercise 200
Solution 9

Solution 10

Solution 11

Solution 12

Solution 13

Solution 14

Solution 15
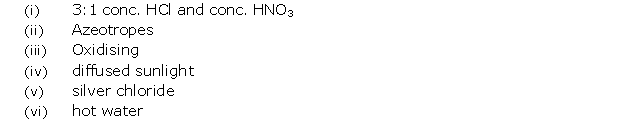
Solution 16
(i) Diffused sunlight
(ii) 22%
(iii) Chlorine
(iv) HCl gas is collected by the downward displacement of air.
Study of Compounds-I: Hydrogen Chloride Exercise 201
Solution 1991-1

Solution 1992-1
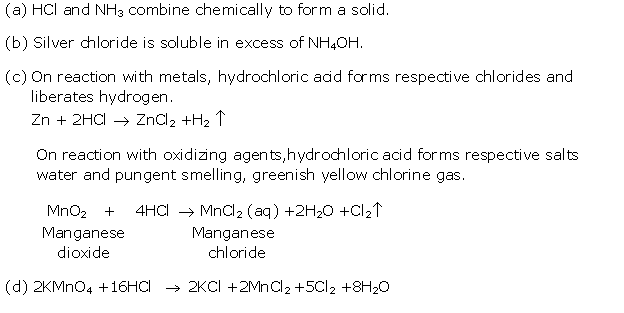
Solution 1992-2
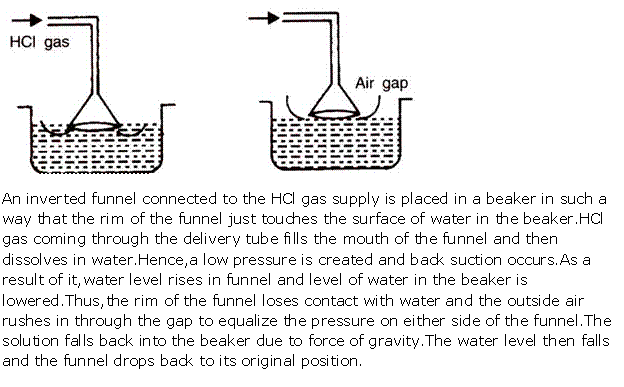
Solution 1992-3

Solution 1994-1
Solution 1995-1
Solution 1996-1

Solution 1997-1
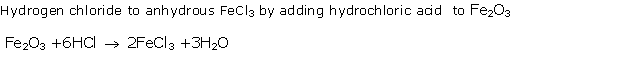
Study of Compounds-I: Hydrogen Chloride Exercise 202
Solution 1998-1
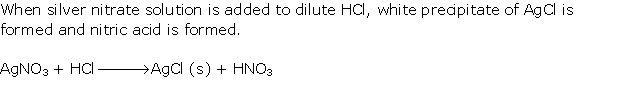
Solution 2000-1

Solution 2000-2

Solution 2000-3
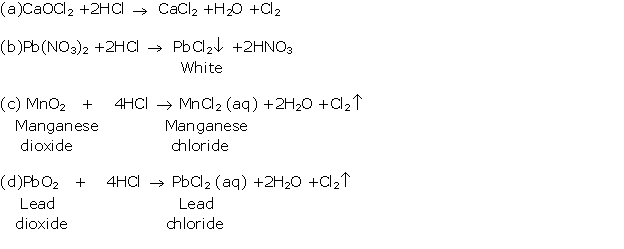
Solution 2000-4
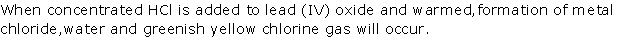
Solution 2001-1
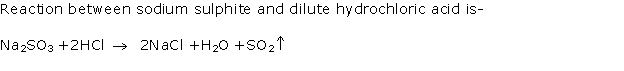
Solution 2001-2

Solution 2001-3
Solution 2002-1

Solution 2002-2

Solution 2004-1
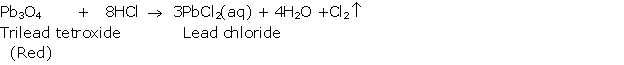
Solution 2004-2
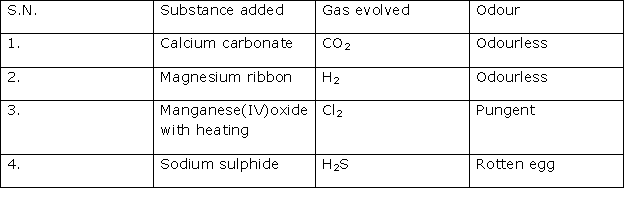
Study of Compounds-I: Hydrogen Chloride Exercise 203
Solution 2004-3

Solution 2005-1
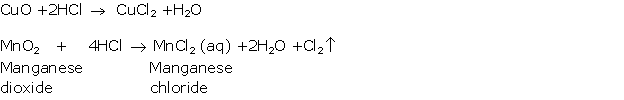
Solution 2005-2
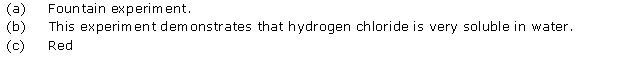
Solution 2006-1
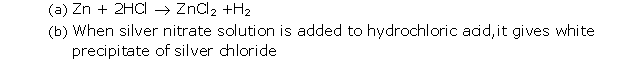
Solution 2007-1

Solution 2008-1

Solution 2009-1
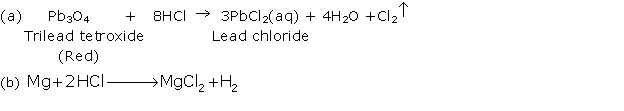
Solution 2009-2

Solution 2010-1
Aqua regia is a mixture of concentrated hydrochloric acid [3 parts] and concentrated nitric acid [1 part].
Study of Compounds-I: Hydrogen Chloride Exercise 204
Solution 2010-2
(i) A = conc. H2SO4 B = NaCl
(ii) ![]()
(iii) When a rod dipped in ammonium hydroxide is brought near the mouth of the gas jar, dense white fumes of ammonium chloride are produced.
(iv) Hydrogen chloride is denser than air.
Solution 2010-3
Silver nitrate solution will give a white ppt. when added to dil. hydrochloric acid, and no change will be observed when it is added to dil. nitric acid.
Solution 2011-1
Being highly soluble in water, hydrogen chloride gas is dried by conc. sulphuric acid.
Solution 2011-2
(i) Diagram to show the arrangement used for the absorption of HCl gas in water:
(ii) Such an arrangement is necessary to prevent back suction of water into the apparatus, and it provides a large surface area for dissolution of hydrogen chloride gas.
(iii) Balanced chemical equations for the laboratory preparation of HCl gas:
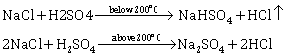
Solution 2013-1
(i) Add silver nitrate solution to both solutions. Sodium chloride will form a curdy white ppt., whereas sodium nitrate will not undergo any reaction.
(ii) Hydrogen chloride gas gives thick white fumes of ammonium chloride when a glass rod dipped in ammonia solution is held near the vapours of the acid, whereas no white fumes are observed in case of hydrogen sulphide gas.
(iii) Ethene gas decolourises the purple colour of KMnO4, whereas ethane does not decolourise KMnO4 solution.
(iv) Calcium nitrate forms no ppt. even with addition of excess of NH4OH, whereas zinc nitrate forms a white gelatinous ppt. which dissolves in excess of NH4OH.
(v) Carbon dioxide gas has no effect on acidified KMnO4 or K2Cr2O7, but sulphur dioxide turns potassium permanganate from pink to colourless.
Solution 2014-1
(i) The gas is HCl (hydrogen chloride) gas.
(ii) Extreme solubility of hydrogen chloride gas is demonstrated by the fountain experiment.
(iii) Ammonia gas is another gas which has the same property which can be demonstrated through this experiment.
Study of Compounds-I: Hydrogen Chloride Exercise 205
Solution 2015-1
(i) Equation for the laboratory preparation of hydrogen chloride gas:
![]()
Although it is a reversible reaction, it goes to completion as hydrogen chloride continuously escapes as a gas.
The reaction can occur up to the stage of the formation of sodium sulphate on heating above 200°C.
![]()
(ii) The drying agent used in the laboratory preparation of hydrochloric acid is conc. sulphuric acid.
The other drying agents such as phosphorus pentoxide (P2O5) and quick lime (CaO) cannot be used because they react with hydrogen chloride.
2P2O5 + 3HCl → POCl3 + 3HPO3
CaO + 2HCl → POCl3 + 3HPO3
(iii) A safety precaution which should be taken during the preparation of hydrochloric acid:
Always wear chemical splash goggles, chemical-
resistant gloves and a chemical-resistant apron in the
laboratory during the preparation of hydrochloric
acid.
Solution 2016-1
(a) HCl turns blue litmus red
Solution 2016-2
(i) When dil. HCl is added to lead nitrate solution and heated, it forms a white precipitate of lead chloride.
Pb(NO3)2 + 2HCl → PbCl2 + 2HNO3
(ii) Dil. HCl reacts with thiosulphate to produce sulphur dioxide, and yellow sulphur is precipitated.
Na2S2O3 + 2HCl → 2NaCl + H2O + SO2 + S ↓
(iii) When dilute hydrochloric acid is added to copper carbonate, it decomposes to give copper chloride.
CuCO3 + 2HCl → CuCl2 + H2O + CO2↑
Solution 2017-1
Dilute hydrochloric acid decomposes iron(II) sulphide to produce iron(II) chloride and hydrogen sulphide having rotten egg smell.
FeS + 2HCl → FeCl2 + H2S
Solution 2017-2
Sulphuric acid precipitates the insoluble sulphate from lead nitrate solution.
![]()
Lead nitrate reacts with hydrochloric acid to give a white ppt. of lead chloride.
![]()