Class 10 FRANK Solutions Chemistry Chapter 12 - Practical Work
Find Frank Solutions for ICSE Class 10 Chemistry Chapter 12 Practical Work at TopperLearning. Learn about the litmus tests for ammonia and water vapour. Go through answers on using sodium hydroxide solution to distinguish zinc nitrate solution from calcium nitrate solution. In addition, revise the differences between lead ion and zinc ion.
Avoid silly mistakes in scoring questions in your ICSE Class 10 Chemistry exam. Practise MCQs, short answer questions and other types of questions in our textbook solutions. Also, make use of our Chemistry practice tests and Selina Solutions to revise chapter-specific questions and answers.
Practical Work Exercise 307
Solution 1

Solution 2

Solution 3

Solution 4

Solution 5

Solution 6
(a) i) Bluish green crystalline solid, on heating, melts to form a bluish green mass and gives off steamy vapours which condense on the cooler part of the test tube.
ii) On further heating, the bluish green mass changes to a black residue.
iii) It gives off a reddish brown gas and gives a gas which rekindles a glowing splinter, i.e. oxygen.
(b) i) A white solid turns yellow on heating.
ii) Gives off a gas which extinguishes a burning wooden splinter.
iii) Gas evolved turns lime water milky.
(c) i) When ammonium chloride is heated in a test tube, the lighter ammonia gas will emerge first and turn a piece of moist red litmus paper blue.
ii) Hydrogen chloride coming up next will change the
litmus paper from blue back to red.
Solution 7
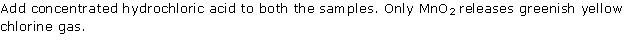
Solution 8

Practical Work Exercise 308
Solution 9

Solution 10

Solution 1999-1

Solution 2000-1

Solution 2001-1
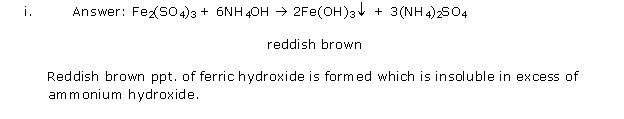
Solution 2001-2
Solution 2002-1
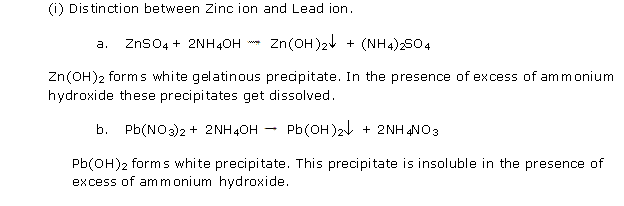
Practical Work Exercise 309
Solution 2003-1

Solution 2004-1

Solution 2004-2
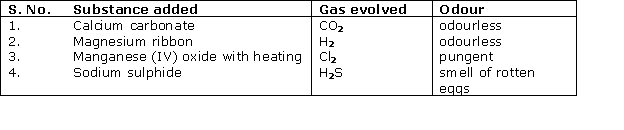
Solution 2005-1
Practical Work Exercise 310
Solution 2006-1

Solution 2006-2

Solution 2007-1

Practical Work Exercise 311
Solution 2008-1

Solution 2010-1
(i) Iron (III) chloride
(ii) Lead (II) chloride
(iii)Nitroso iron (II) sulphate
(iv)Sodium chloride
(v) Chromium sulphate
Solution 2011-1
On addition of ammonium hydroxide in a small quantity, a blue-coloured copper hydroxide precipitate is formed. This copper hydroxide of light blue colour dissolves in excess of ammonium hydroxide to yield a deep blue solution.
Solution 2011-2
(i) D
(ii) C
(iii) E
(iv) A
(v) F
(vi) B
Practical Work Exercise 312
Solution 2013-1
(i) Lead nitrate decrepitates on heating; a yellow solid is formed and it fuses with glass. Lead nitrate decomposes to lead oxide, nitrogen dioxide and oxygen.
(ii) Oxygen is evolved.
2KNO3→ 2KNO2 + O2
Solution 2013-2
(i) Add silver nitrate solution to both solutions. Sodium chloride will form a curdy white ppt., whereas sodium nitrate will not undergo any reaction.
(ii) Hydrogen chloride gas gives thick white fumes of ammonium chloride when a glass rod dipped in ammonia solution is held near the vapour of the acid, whereas no white fumes are observed in case of hydrogen sulphide gas.
(iii) Calcium nitrate forms no ppt. even with addition of excess of NH4OH, whereas zinc nitrate forms a white gelatinous ppt. which dissolves in excess of NH4OH.
(iv) Carbon dioxide gas has no effect on acidified KMnO4 or K2Cr2O7, but sulphur dioxide turns potassium permanganate from pink to colourless.
Solution 2013-3
Sulphuric acid precipitates the insoluble sulphate of barium from the solution of barium chloride.
BaCl2 + H2SO4→ BaSO4 + 2HCl
Dilute HCl does not react with barium chloride solution, and thus, no precipitate is produced in the reaction.
Solution 2014-1
(i) On carrying out the flame test with a salt P, a brick red flame is obtained. Hence, the cation P is Ca2+.
(ii) A gas Q turns moist lead acetate paper silvery black. Hence, the gas is H2S.
(iii) pH of liquid R is 10. Hence, substance R is a base.
(iv) Salt S is prepared by reacting dilute sulphuric acid with copper oxide. Hence, salt S is copper sulphate.
Solution 2014-2
(i) Sodium carbonate crystals on reaction with dilute HCl form sodium chloride, water and carbon dioxide, which is evolved with brisk effervescence. This is a neutralisation reaction as sodium carbonate is a basic salt, while hydrochloric acid is an acid. The chemical equation for this reaction is as follows:
Na2CO3 + 2HCl → 2NaCl +H2O + CO2
(ii) Calcium nitrate solution on reaction with excess of sodium hydroxide produces calcium hydroxide and sodium nitrate. Calcium nitrate reacts with excess of sodium hydroxide to form a white precipitate of calcium hydroxide, which is sparingly soluble, and colourless sodium nitrate. The reaction is as follows:
Ca(NO3)2 + 2NaOH → Ca(OH)2 + 2NaNO3
(iii) Acidified aqueous copper sulphate solution is electrolysed with copper electrodes by electrolysis. The electrolysis of an aqueous solution of copper sulphate using copper electrodes (i.e. using active electrodes) results in the transfer of copper metal from the anode to the cathode during electrolysis. Copper sulphate is ionised in aqueous solution.
Chemical equation:
CuSO4 → Cu2+ + SO42-
The positively charged copper ions migrate to the cathode, where each gains two electrons to become copper atoms which are deposited on the cathode.
Cu2+ + 2e- → CuHence, the colour of copper sulphate changes from blue to colourless.
(iv) When ammonium chloride is heated with calcium hydroxide, ammonia gas is released.
2NH4Cl + Ca(OH)2→ CaCl2 + 2NH3 + 2H2O
The liberated gas turns red litmus blue.
(v) When moist starch iodide paper is introduced into chlorine gas, chlorine oxidises iodide to iodine, which shows up as blue when it forms a complex with starch.
Solution 2015
(i) Zn2+
(ii) Cu2+
(iii) Ca2+
(iv) NH4+
Practical Work Exercise 313
Solution 2015-2
(i) Chloride ion (Cl-)
(ii) Carbonate (CO32-)
(iii) Sulphate (SO42-)
Solution 2015-3
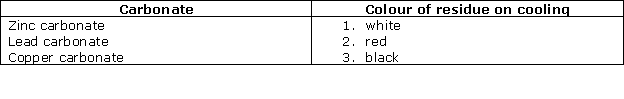
(i) A deliquescent salt: MgCl2
(ii) An insoluble chloride: AgCl
(iii) On heating, this salt gives a yellow residue when hot and a white residue when cold: ZnCO3
(iv) On heating this salt, a brown-coloured gas is evolved: Ca(NO3)2
Solution 2015-4
(i) 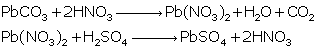
(ii) ![]()
(iii) ![]()
Solution 2016-1
(i) Fe3+ ion
![]()
(ii) Pb2+ ion
![]()
(iii) Ca2+ ion
![]()
Solution 2016-2
(i) Sulphur dioxide
Freshly prepared K2Cr2O7 paper changes from orange to green.
(ii) Hydrogen sulphide
The gas released has a rotten egg smell.
Solution 2017-1
(i) KCl
(ii) ZnCO3

