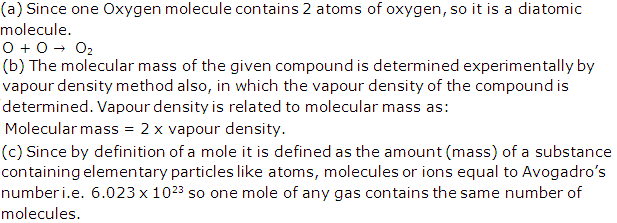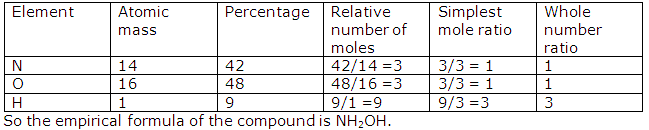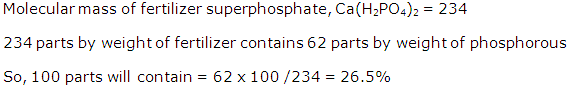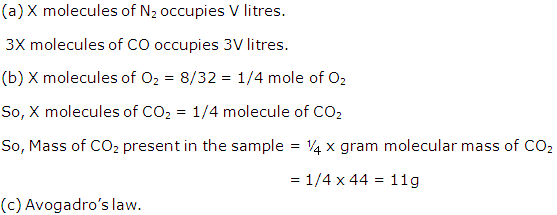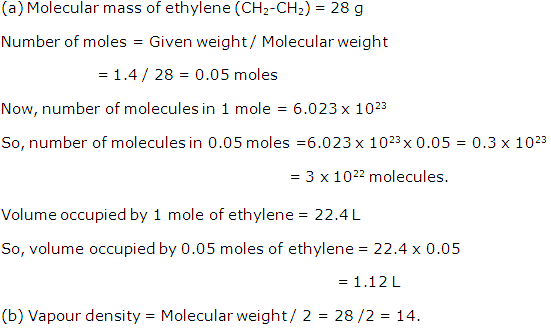Class 10 FRANK Solutions Chemistry Chapter 5 - Mole Concept And Stoichiometry
Practise Chemistry concepts with Frank Solutions for ICSE Class 10 Chemistry Chapter 5 Mole Concept and Stoichiometry. Revise Gay-Lussac’s law of combining volumes and Avogadro’s law. Through our Frank Chemistry solutions, learn to calculate the relative molecular masses of chloroform, sodium acetate, ammonium sulphate and potassium chlorate.
In addition, TopperLearning’s ICSE Class 10 Chemistry Frank Solutions will also help you learn the usage of the empirical formula and the molecular formula. You can also practise these chapter concepts using our online Selina Solutions and other Chemistry learning resources.
Mole Concept And Stoichiometry Exercise 115
Solution 14
Solution 1
(a) Gay-Lussac's law: It states that 'when gases react, they do so in volumes which bear a simple ratio to one another, and also to the volume of the gaseous product, provided all the volumes are measured at the same temperature and pressure'.
(b) Avogadro's law : It states that 'Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules'.
Solution 2
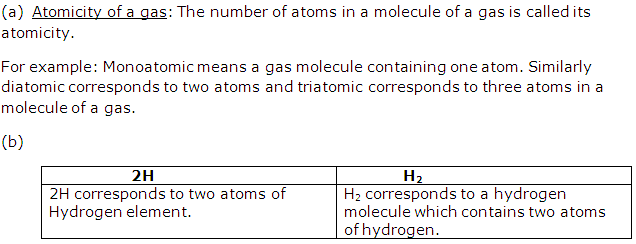
Solution 3
Solution 4
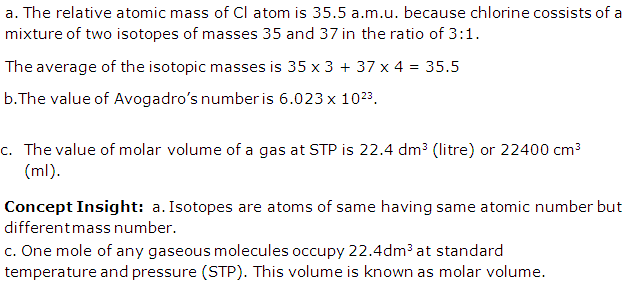
Solution 5

Solution 6

Solution 7
(a) Gram atom: "The quantity of the element which weighs equal to its gram atomic mass is called one gram atom of that element".
For example: The gram atomic mass of hydrogen is 1g. So, 1g of hydrogen is 1 gram atom of hydrogen.
(b) Gram mole: "A sample of substance with its mass equal to its gram molecular mass is called one gram molecule of this substance or one gram mole".
For example: Gram molecular mass of oxygen is 32 g. So One gram mole of oxygen is 32g.
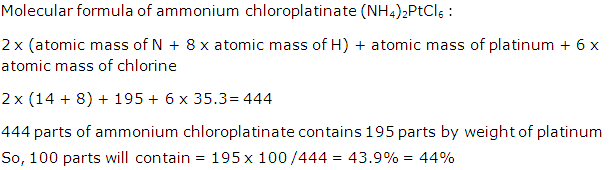
Solution 8

Solution 9
Molecular formula: "Molecular formula of a compound denotes the actual number of atoms of different elements present in one molecule of the compound".
Solution 10
(b) The empirical formula of C6H12O6 is: CH2O.
(c) The empirical formula of C2H2 is: CH
(d) The empirical formula of CH3COOH is: CH2O.
Solution 11
Three pieces of information conveyed by the formula H2O is that:
It shows that there are 2hydrogen atoms and 1oxygen atoms present in H2O.
The hydrogen and oxygen atoms are present in simplest whole number ratio of 2:1.
It represents one molecule of compound water.
Solution 12
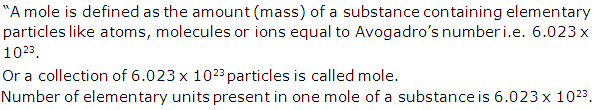
Solution 13
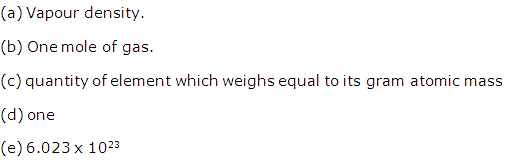
Solution 15
(b) C6H12O6.
Mole Concept And Stoichiometry Exercise 116
Solution 16

Solution 17
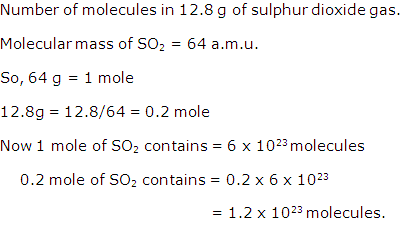
Solution 18
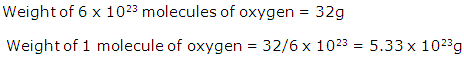
Solution 19
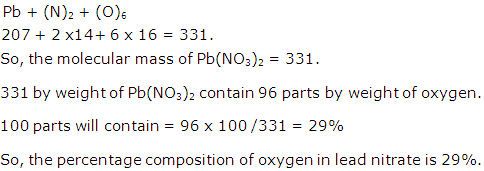
Solution 20
Solution 21
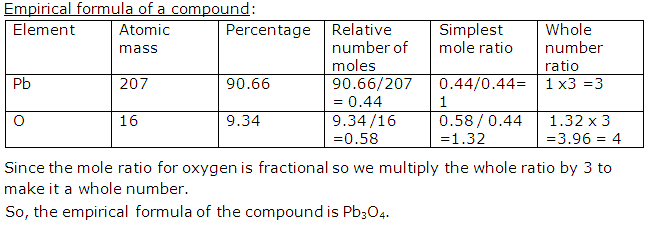
Solution 22
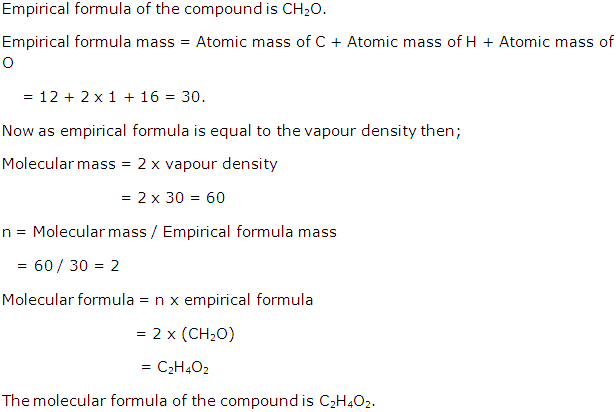
Solution 23
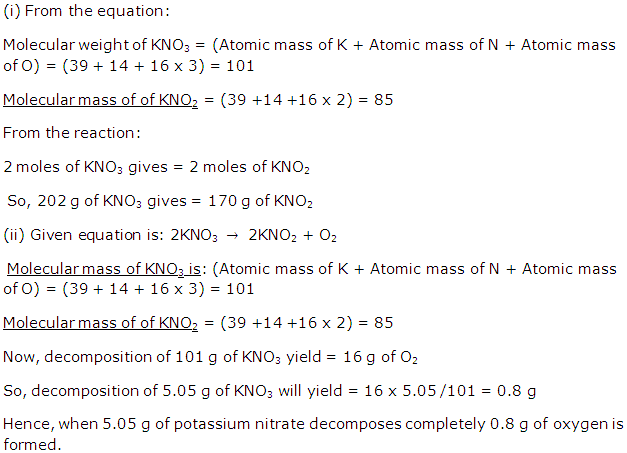
Solution 24

Solution 25
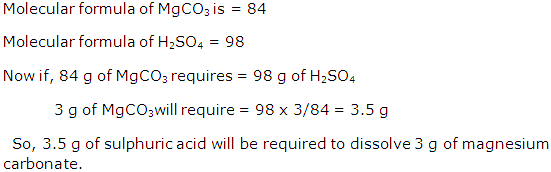
Solution 26

Solution 27
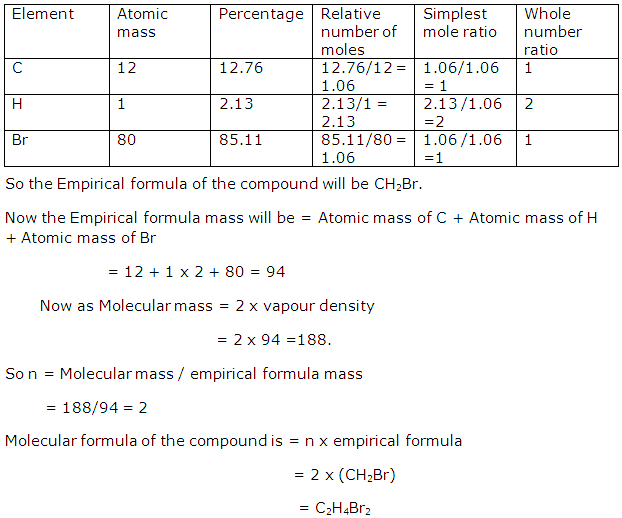
Solution 28
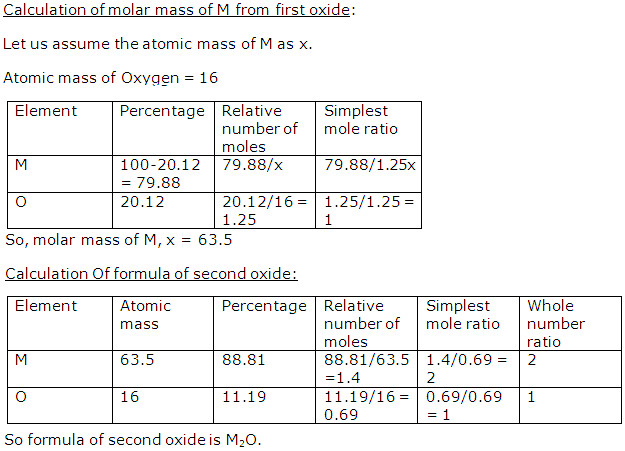
Solution 29

Solution 30
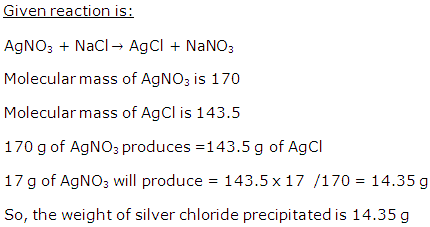
Solution 31
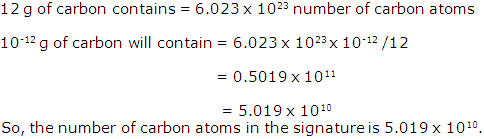
Mole Concept And Stoichiometry Exercise 117
Solution 1996-1

Solution 1996-2

Solution 1996-3
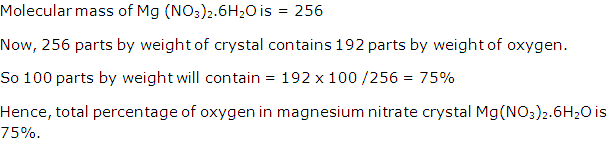
Solution 1996-4
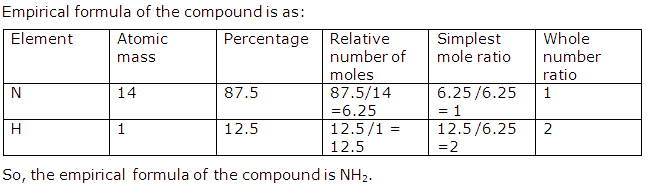
Solution 1997-1

Solution 1997-2
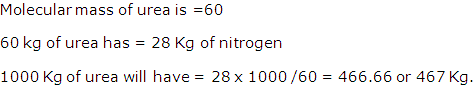
Solution 1997-3
Solution 1997-4
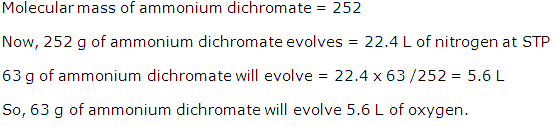
Solution 1998-1
Mole Concept And Stoichiometry Exercise 118
Solution 1998-2

Solution 1999-1

Solution 1999-2

Solution 1999-3

Solution 2000-1

Solution 2000-2
Mole Concept And Stoichiometry Exercise 119
Solution 2001-1
Solution 2001-2
According to Gay-Lussac's law:
2 vol. of C2H6 requires= 7 vol. of oxygen
Vol. of C2H6 = 2 vol. = 100 L
Vol. of oxygen required = 7 vol. =350 L
Solution 2001-3
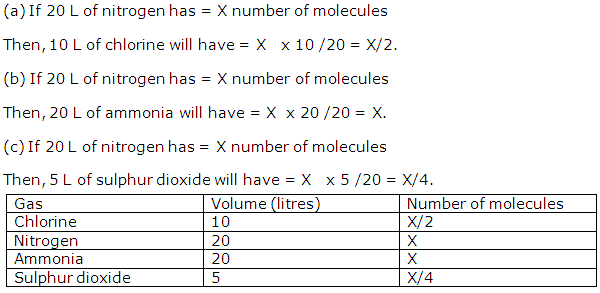
Solution 2001-4
Solution 2001-5
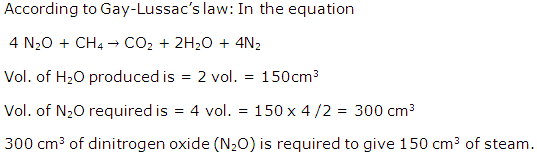
Solution 2001-6
Solution 2001-7
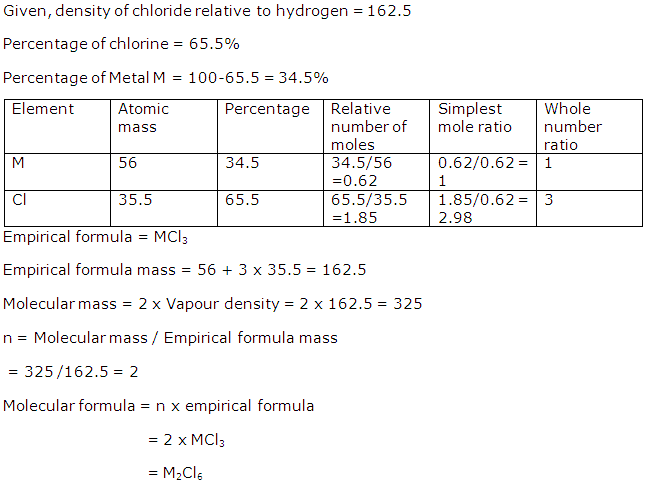
Solution 2002-1
Solution 2002-2
Solution 2002-3
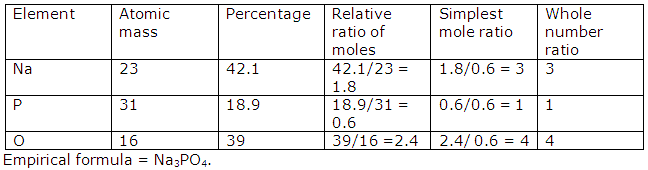
Mole Concept And Stoichiometry Exercise 120
Solution 2003-1

Solution 2004-1

Solution 2004-2
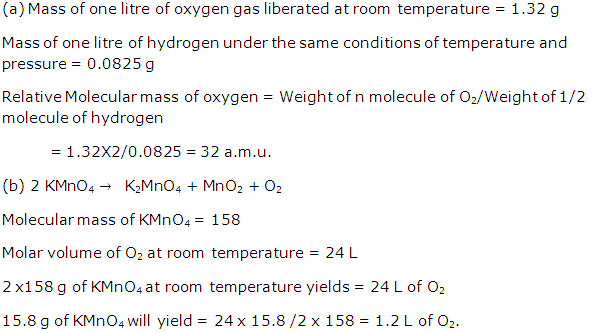
Solution 2005-1
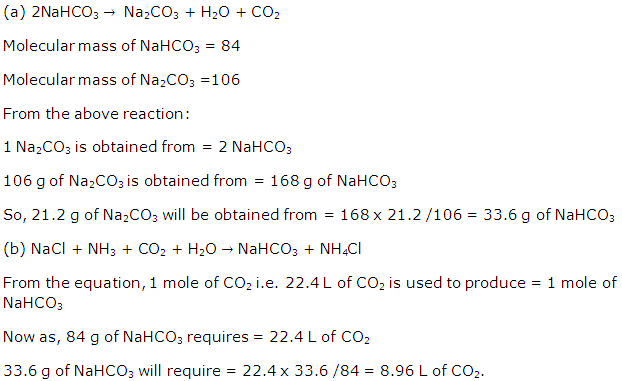
Mole Concept And Stoichiometry Exercise 121
Solution 2006-1

Solution 2006-2
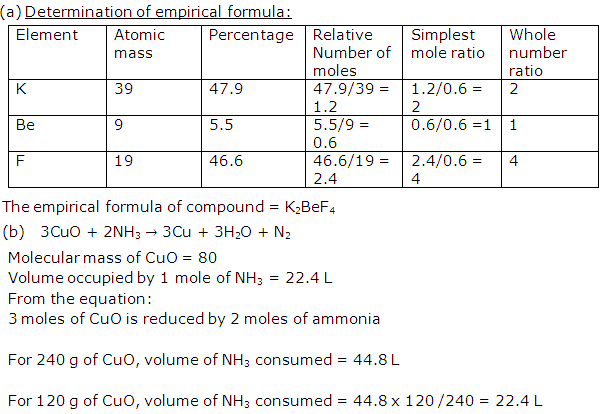
Solution 2006-3
Solution 2006-4

Solution 2007-1

Mole Concept And Stoichiometry Exercise 122
Solution 2007-2
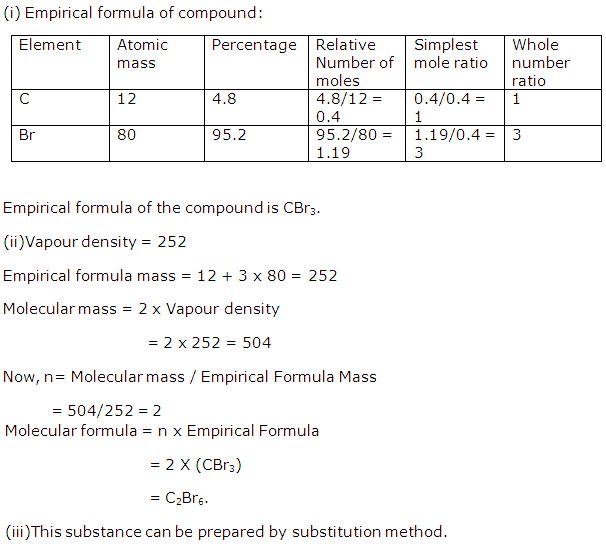
Solution 2008-1
Solution 2008-2
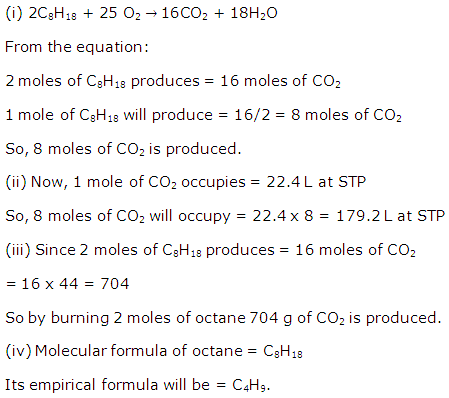
Solution 2008-3
Mole Concept And Stoichiometry Exercise 123
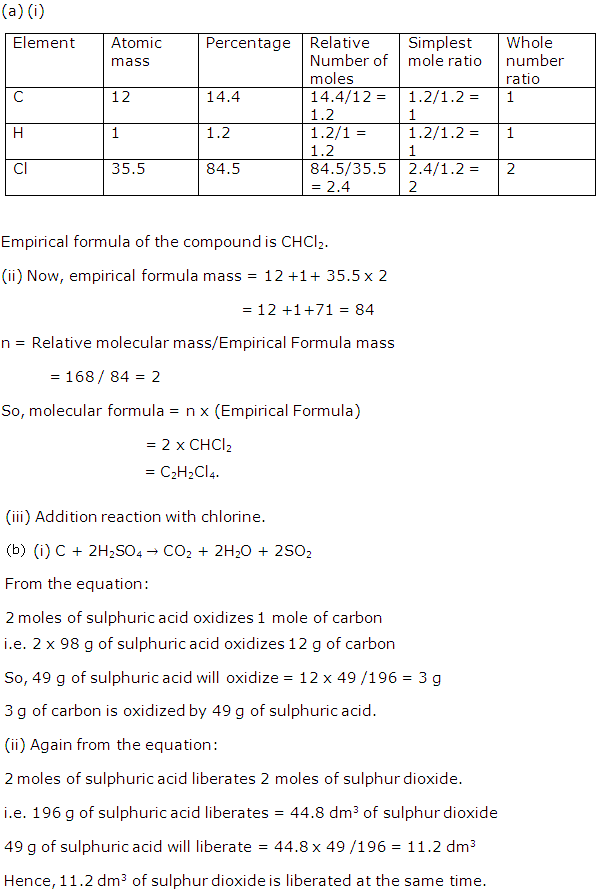
Solution 2009-1
Solution 2009-2
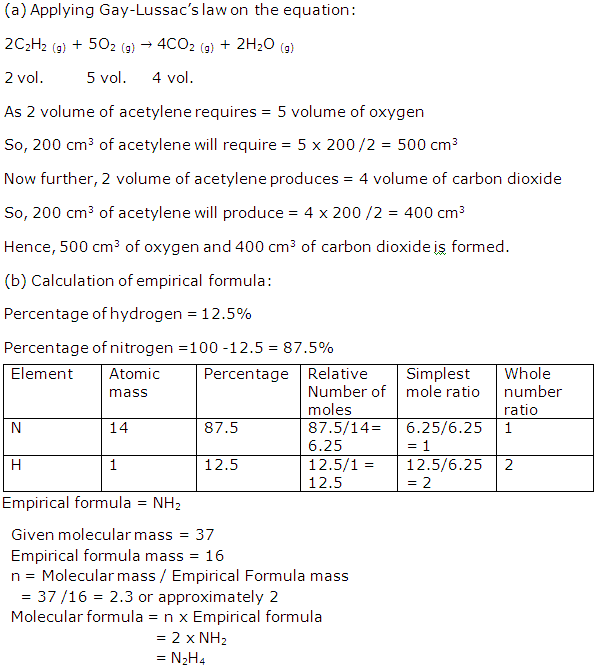
Solution 2009-3
Solution 2009-4
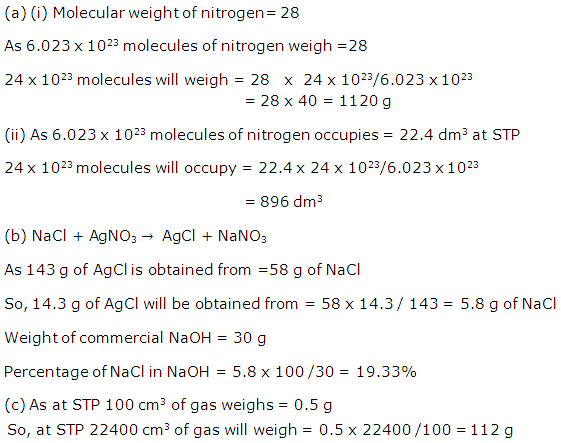
Solution 2010-1

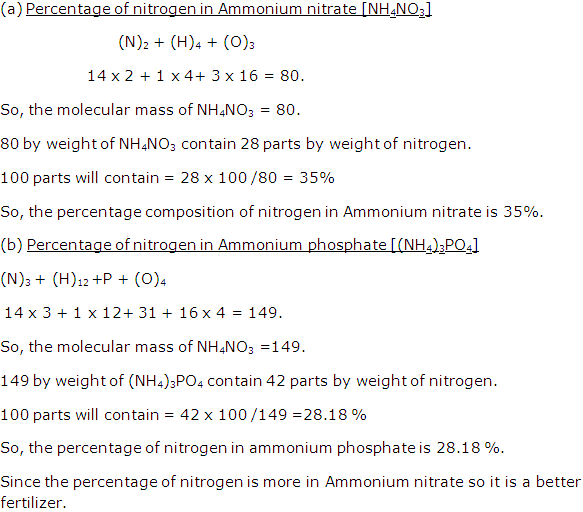
(ii) Molecular mass of NH4(NO3) = 80
H = 1, N = 14, O = 16
% of nitrogen
As 80 g of NH4 (NO3) contains 28 g of nitrogen,
![]()
% of nitrogen
As80 g of NH4(NO3) contains48 g of oxygen
![]()
Solution 2010-2
(i) Equation for the reaction of calcium carbonate with dilute hydrochloric acid:
![]()
(ii) Relative molecular mass of calcium carbonate=100
Mass of 4.5 moles of calcium carbonate
= No. of moles× Relative molecular mass
= 4.5×100
= 450g
(iii) ![]()
As100g of calcium carbonate gives 22.4dm3 of CO2,
![]()
(iv) Molecular mass of calcium carbonate =100
Relative molecular mass of calcium chloride =111
As 100 g of calcium carbonate gives 111g of calcium chloride,
![]()
(v) Molecular mass of HCl=36.5
Molecular mass of calcium carbonate =100
As 100 g of calcium carbonate gives (2×36.5)= 73g of HCl,
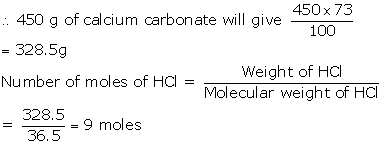
Mole Concept And Stoichiometry Exercise 124
Solution 2011-1
(i) Atomic mass: S = 32 and O = 16
Molecular mass of SO2=32+(2×16)=64g
As 64 g of SO2 = 22.4dm3,
![]()
(ii) Gay-Lussac's law: When gases react, they do so in volumes which bear a simple ratio to one another and to the volume of the gaseous product, if all the volumes are measured at the same temperature and pressure.
(iii) C3H8 + 5O2 → 3CO2 + 4H2O
Molar mass of propane = 44
44 g of propane requires 5 × 22.4 litres of oxygen at STP.
![]()
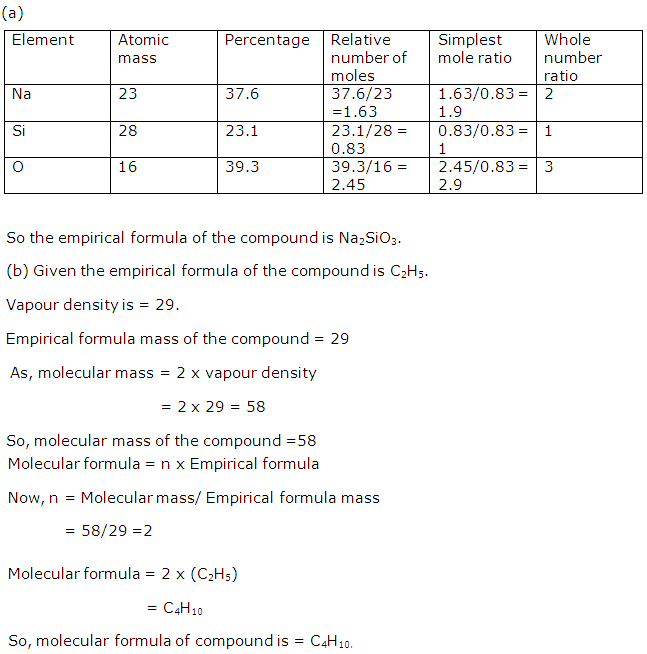
Solution 2011-2
|
Element |
Relative atomic mass |
% Compound |
Atomic ratio |
Simplest ratio |
|
H |
1 |
2.13 |
2.13/1 = 2.13 |
2 |
|
C |
12 |
12.67 |
12.67/12 = 1.055 |
2 |
|
Br |
80 |
85.11 |
85.11/80 = 1 |
1 |
Empirical formula = CH2Br
n(Empirical formula mass of CH2Br) = Molecular mass (2 × VD)
n(12 + 2 + 80) = 94 × 2
n = 2
Molecular formula = Empirical formula × 2
= (CH2Br) × 2
= C2H4Br2
Solution 2011-3
(i) 1022 atoms of sulphur
6.022 × 1023 atoms of sulphur will have mass = 32 g
![]()
(ii) 0.1 mole of carbon dioxide
1 mole of carbon dioxide will have mass = 44 g
0.1 mole of carbon dioxide will have mass = 4.4 g
Solution 2013-1

Solution 2013-2

Solution 2014-1
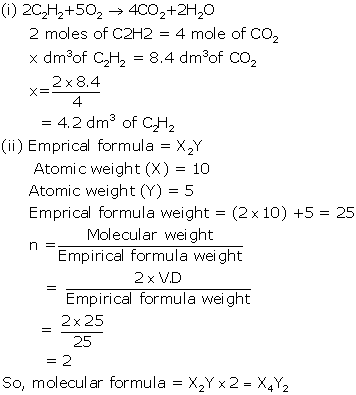
Mole Concept And Stoichiometry Exercise 125
Solution 2014-2
(i) Vapour density
(ii) Ionisation
Solution 2014-3
(i) Avogadro's law:Equal volumes of all gases under similar conditions of temperature and pressure contain the same number of molecules.
(ii)

Solution 2015-1
6.02 × 1023 atoms of carbon
Solution 2015-2
(i) 3.2 g of S has number of atoms = 6.023 × 1023 x 3.2 /32
= 0.6023 × 1023
So, 0.6023 × 0.6023 ×1023/6.023 × 1023 = 4g
(ii) 6 litres of hydrogen and 4 litres of chlorine when mixed result in the formation of 8 litres of HCl gas.
When water is added to it, it results in the formation of hydrochloric acid. Chlorine acts as a limiting agent leaving behind only 2 litres of hydrogen gas.
Therefore, the volume of the residual gas will be 2 litres.
(iii)
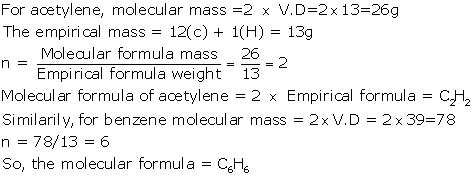
Solution 2015-3
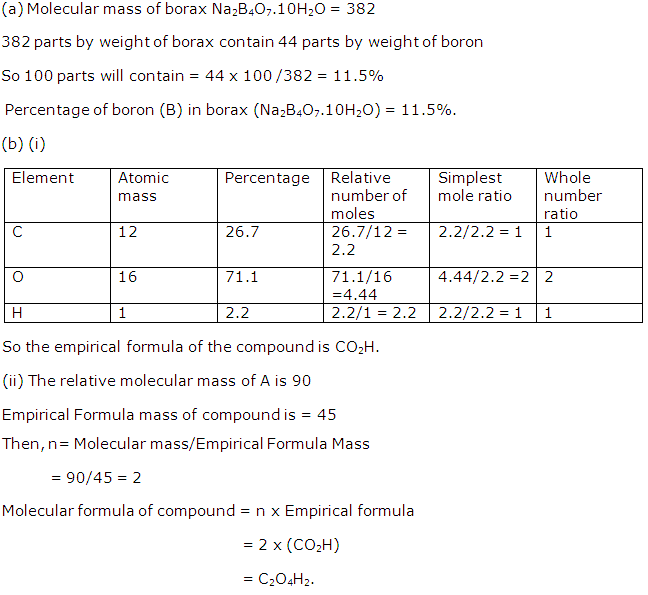
(i)

(ii) 252 g of ammonium dichromate gives 22.4 dm3of N2
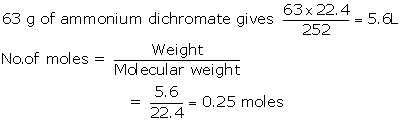
(iii)

(iv) 252 g of ammonium dichromate gives 152 g of CrO3
![]()
Solution 2016-1
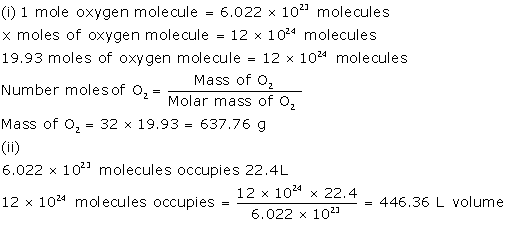
Solution 2016-2
% of carbon = 82.76%
% of hydrogen = 100 - 82.76 = 17.24%
|
Element |
% Weight |
Atomic Weight |
Relative No. of moles |
Simplest Ratio |
|
C |
82.76 |
12 |
82.76/12 = 6.89 |
6.89/6.8 = 1 × 2 = 2 |
|
H |
17.24 |
1 |
17.24/1 = 17.24 |
17.24/6.89 = 2.5 × 2 = 5 |
Empirical formula = C2H5
Empirical formula weight = 2 × 12 + 1 × 5 = 24 + 5 = 29
Vapour density = 29
Relative molecular mass = 29 × 2 = 58
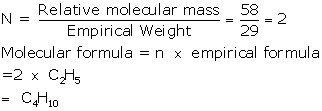
Mole Concept And Stoichiometry Exercise 126
Solution 2016-3
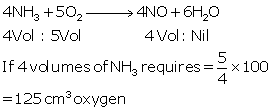
Solution 2016-4
Mass of gas = 32 g
Volume occupied by 32g of gas = 20litres
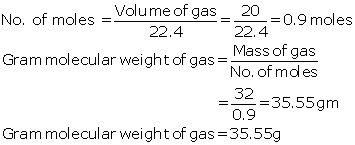
Solution 2016-5
2Ca(NO3)2→ 2CaO + 4NO2 + O2
Molecular weight of 2Ca(NO3)2 = 2[40+2(14+48) = 32g
Molecular weight of CaO = 2(40 + 16) = 112g
328 g of Ca(No3]2 liberates 4 moles of NO2
328 g of Ca (NO3)2 liberates 4 × 22.4 L of NO2
![]()
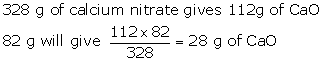
Solution 2016-6
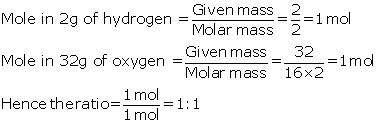
Solution 2017-1
(a) 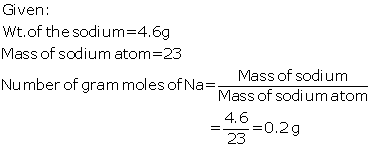
(b) 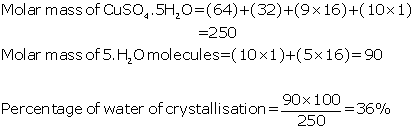
(c) ![]()

Solution 2017-2
(a)
Given:
C3H8 + 5O2 → 3CO2 + 4H2O
Volume of air = 1000 cm3
Percentage of oxygen in air = 20%
From the given information,
![]()
According to Gay-Lussac's law,
1 vol. of propane consumes 5 vol. of oxygen.
Volume of oxygen = 1000 cm3 × 20% = 200 cm3
Therefore,
Volume of propane burnt for every 200 cm3 of oxygen,
![]()
40 cm3of propane is burnt.
(b)
(i) Given:
Volume of gas at STP = 11.2 litres
Mass of gas at STP = 24 g
Gram molecular mass = ?
Mass of 22.4 L of a gas at STP is equal to its gram
molecular mass.
11.2 L of the gas at STP weighs 24 g
So,
22.4 L of the gas will weigh
![]()
Gram molecular mass = 48 g
Solution 2017-3
(a)
Given:
Mass of hydrogen = 1 kg at 298 K and 1 atm pressure
Moles of hydrogen =?
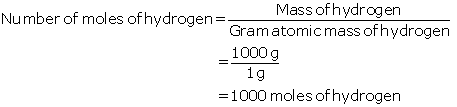
(b) Molecular mass of CO2 = 12 + 2 × 16 = 44g
So, vapour density (VD) = mol. Mass/2 = 44/2 = 22
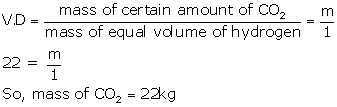
(c) According to Avogadro's law, equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules.
(d) So, number of molecules of carbon dioxide in the cylinder = number of molecules of hydrogen in the cylinder = X