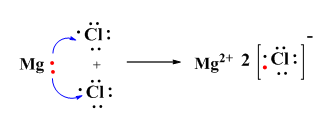Class 10 FRANK Solutions Chemistry Chapter 2 - Chemical Bonding
What is a chemical bond? How to draw the electron–dot structure of NaCl? Find answers in Frank Solutions for ICSE Class 10 Chemistry Chapter 2 Chemical Bonding. The Frank Chemistry solutions at TopperLearning cover examples of solid covalent compounds, gaseous non-polar compounds and more.
In these ICSE Class 10 Chemistry chapter solutions, you will also learn about the structures of ammonium and hydronium ions. You may also check our video lessons, Selina Solutions and practice tests to revise this Chemistry chapter further.
Chemical Bonding Exercise 42
Solution 1
Chemical bonding: The phenomenon during which a chemical bond is formed is called chemical bonding.
Solution 2
Solution 3
For example: Sodium chloride (NaCl).
Covalent compounds: The chemical compound, formed as a result of mutual sharing of electrons or electron pairs thereby establishing a covalent bond is called a covalent or molecular compound.
For example: Hydrogen molecule (H2)
Solution 4
1) Low ionization energy of electropositive atom
2) High electron affinity of the electronegative atom.
3) Large electronegativity difference.
4) High lattice energy.
Concept Insight: 1) Lower is the ionization energy of atom, higher is its tendency to lose electron to form a cation and form ionic bond.
2) Higher the value of electron affinity of an atom, greater will be its tendency to form anion and form ionic bond.
3) If the electronegativity difference of two elements is higher, more easy will be the transfer of electrons and hence more chances of ionic bond formation.
4) Lattice energy is the energy released when positive and negatively charged atoms called ions come closer to form a crystal because the attractive forces among the oppositely charged ions tend to decrease the energy of the system. Higher is the lattice energy, greater will be the ease of formation of the compound.
Solution 5
| Ioniccompounds | Covalent compounds |
| These contain electrovalent or ionic bond. | These contain covalent bond. |
| They are generally crystalline solids. | They are generally volatile liquids or gases. |
| They have high melting and boiling points. | They have low melting and boiling points. |
| They are bad conductors of electricity in solid state but are good conductors in the molten or aqueous states. | They do not conduct electricity. |
| They are generally soluble in water but insoluble in organic solvents. | They are generally soluble in organic solvents but insoluble in water. |
| They consist of ions. So, their reactions in solutions are fast. | They consist of molecules. So, their reactions in solutions are slow. |
Solution 6
While methane has a non polar covalent bond because in case of methane molecule the shared electron pairs are at equal distance from the carbon and hydrogen atoms, because neither the carbon atom nor the hydrogen atom has enough electronegativity difference between each other to attract the shared pairs of electrons towards itself. Hence no charge separation occurs in the covalent bond due to which it is called non polar covalent bond.
Concept Insight: When a covalent bond is formed between the atoms of the same elements of equal electronegativity then the electron pairs are shared equally between the atoms and the bond so formed is called non polar covalent bond. On the other hand, if the covalent bond is formed between atoms of different elements, with difference in electro negativity, the electrons are not shared equally between the atoms. The more electronegative atom pulls the bonded pair of electrons towards itself and acquires negative charge while the other less electro negative atom acquires positive charge and the bond becomes polar covalent bond.
Solution 7
During formation of ionic bond one atom undergoes oxidation while another atom undergoes reduction.
Chemical Bonding Exercise 43
Solution 8
Solution 9
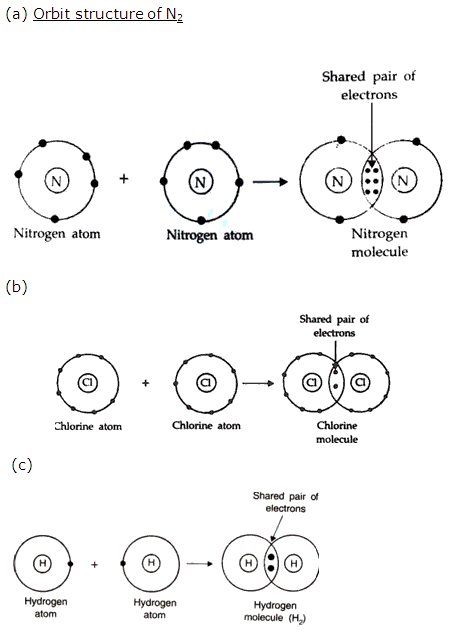
Solution 10
On the other hand, carbon tetra chloride has non polar covalent bond and water has polar covalent bond. Hence, water is unable to break the non polar covalent bond of carbon tetra chloride. So it is insoluble in water
(b) Helium does not form He2 molecule as it has its outermost shell complete i.e. two electrons in its valence shell. Due to this complete valence shell helium atom is very stable hence does not participate in chemical bonding to form He2 molecule.
(c) Pure water does not conduct electricity because it has a polar covalent molecule hence does not have ions in it which can conduct electricity.
On adding sodium chloride to pure water, sodium chloride breaks apart into sodium and chloride ions because water being polar decreases the strong forces of attraction among sodium and chloride ions. Now, pure water has ions present in it which can conduct electricity.
(d) Cl2 is a non polar molecule because the bond is between same atoms that is chlorine with zero electronegativity difference among them. So the shared electron pair is attracted equally by the two chlorine atoms hence there is no separation of charges in the bond formed so the chlorine molecule is non polar.
In case of HCl the bond is formed between two different atoms that is hydrogen and chlorine with enough electro negativity difference so that the shared electron pair is attracted towards more electronegative chlorine atom which acquires partial negative charge while the hydrogen atom acquires partial positive charge hence HCl is a polar molecule.
(e) Metals have low ionization energy due to which they can lose their outermost electrons easily to form positive metallic ions hence metals are electropositive.
For example Sodium metal always form Na+ ions, Potassium forms K+ ions etc.
Solution 11
(ii) When the electro negativity difference between the two atoms is low then the bond formed will be polar covalent bond.
(iii) When the electro negativity difference between the two atoms is zero then the bond formed will be purely covalent.
(b) Ionic compounds = NO, NH4Cl, NH4NO3
Covalent compounds = N2, NH3, NO
Polar compounds = NCl3
Concept Insight: Electro negativity difference between the bonded atoms determines the ease of transfer of electrons between the atoms. On the basis of extent of transfer of electrons between the two atoms the bond will be ionic, covalent or polar.
Solution 12
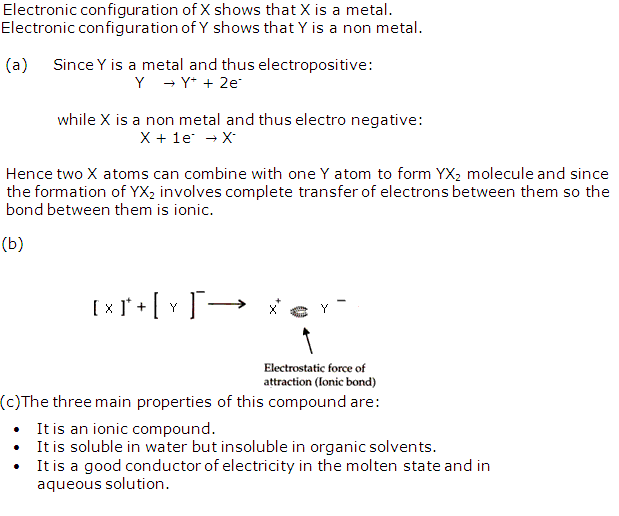
Solution 13
(b) Urea, Glucose.
(c) CH4, benzene.
(d) SO2, H2S
(e) H2, N2
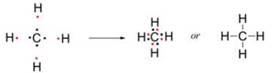
Solution 14
The necessary conditions for the formation of covalent molecule are:
Number of valence electrons: Both the participating atoms should have four or more valence electrons in their valence shell.
Equal electro negativities: The combining atoms should have equal electro negativities so that no transfer of electrons takes place.
Equal electron affinities: The combining atoms should also have equal electron affinities i.e. equal attraction for electrons.
Ionization energy: It should be high for both the atoms so that there is no chance of removal of electrons.
High nuclear charge and small inter nuclear distance: Both these conditions favor the formation of covalent bond because during the formation of a covalent bond the electron density gets concentrated between the nuclei of the combining atoms and this electronic charge is responsible for holding the two nuclei together.
The properties of covalent compounds are:
Nature: They are generally volatile liquids or gases. Some may be gases like urea, sugar etc.
Low melting and boiling points: Since the intermolecular forces of attraction are weak, very small amount of heat energy is required to overcome these forces hence their melting and boiling points are low.
Electrical conductivity: Since covalent compounds are made up of molecules and not ions, so they do not conduct electricity.
Solubility: These are insoluble in water but soluble in organic solvents.
Ionization in solution: These do not ionize when dissolved in water except some polar covalent compounds like HCl.
Molecular reactions: These participate in reactions as a molecule so the reactions are called molecular reactions. These are slow reactions.
Solution 15
Coordinate bond: The bond formed between two atoms by a pair of electrons, provided entirely by one of the combining atoms, is called a coordinate bond or dative bond.
Conditions for the formation of coordinate bond:
One of the two atoms must have at least one lone pair of electrons.
Another atom should be short of at least a lone pair of electrons.
Solution 16
For example in NH3, Nitrogen has a lone pair of electrons which is not shared with any hydrogen atom.
Shared pair: A pair of electrons which is shared with other atoms to form a bond is known as shared pair of electrons.
For example in HCl the pair of electrons responsible for bond formation between H and Cl is called shared pair.
Solution 17
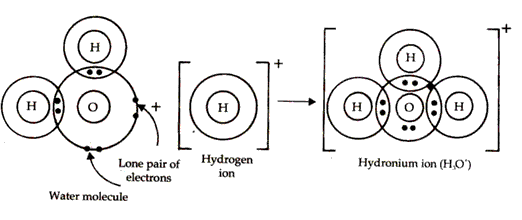
Oxygen atom in water molecule attains an octet by forming two single covalent bonds with two hydrogen atoms but it still contains two lone pairs of electrons.
H3O+ is formed when the oxygen atom in water molecule donates a lone pair of electrons to a hydrogen ion thus establishing a coordinate bond between the oxygen atom and the hydrogen ion.
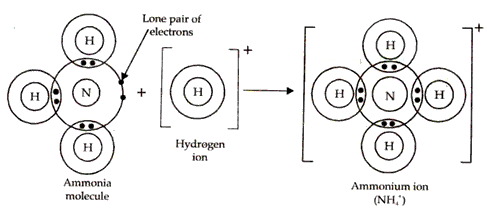
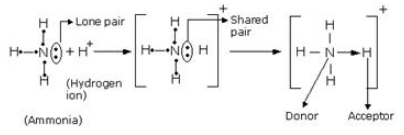
(b) Structure of Ammonium ion: Ammonium ion (NH4+) is formed when a hydrogen ion or proton combines with ammonia molecule. Nitrogen atom in ammonia has a lone pair of electrons on it which it shares with the hydrogen ion which tends to acquire the configuration of helium atom. Thus, a co-ordinate bond results between nitrogen atom and hydrogen ion in ammonium ion.
Solution 18
(b)Covalent compounds have low melting and boiling point because the intermolecular forces of attraction among the molecules of covalent compounds are weak. Hence very small amount of heat energy is required to overcome the attraction between the molecules.
(c) On the basis of principle like dissolves like we can interpret the insolubility of non polar covalent compounds. Since water is a polar covalent compound that is it has positively and negatively charged ends but the non-polar covalent compounds do not have any kind of charge separation. So water molecules are unable to interact with the molecules of non polar compound and break apart the intermolecular forces of attraction among non-polar molecules making them soluble in water.
(d) Polar covalent compounds are good conductors of electricity because when these are dissolved in water, they ionize and act as electrolyte to produce ions which are responsible for conduction of electricity.
For example polar covalent compound HCl in water behaves as:
These hydronium and chloride ions produced on dissolution of HCl in water are responsible for conduction of electricity.
Solution 19
(a) The electrovalent bond or ionic bond is called heteropolar bond.
(b) When ionic compounds are dissolved in water, their constituent ions get separated; this phenomenon is called ionisation or dissociation.
(c) Ionic compounds generally are soluble in water and insoluble in organic solvents.
(d) Coordinate bond is also called dative bond.
(e) A coordinate bond is represented by an arrow pointing from donor to acceptor atom.
(f) Four single covalent bonds are formed between the carbon and chlorine atoms.
Chemical Bonding Exercise 44
Solution 20
(i) (d) High lattice energy
(ii) (b) Low boiling and low melting points
(iii) (a) Equal electro negativities of combining atoms
(iv) (d) NH4⁻
(v) (b) Methane
Solution 2002-1
Chemical Bonding Exercise 45
Solution 2004-1
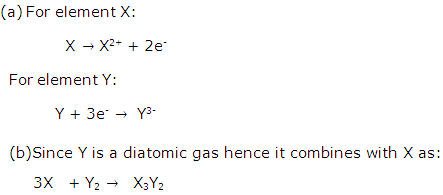
Solution 2005-1
Solution 2006-1
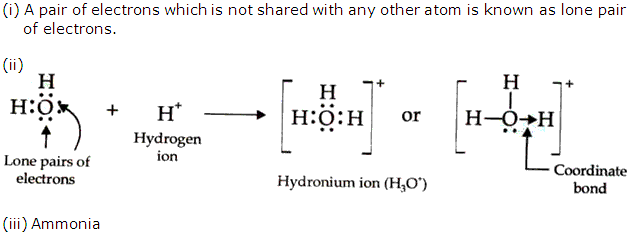
Solution 2006-2
(ii) (a)
Chemical Bonding Exercise 46
Solution 2007-1
Solution 2008-1
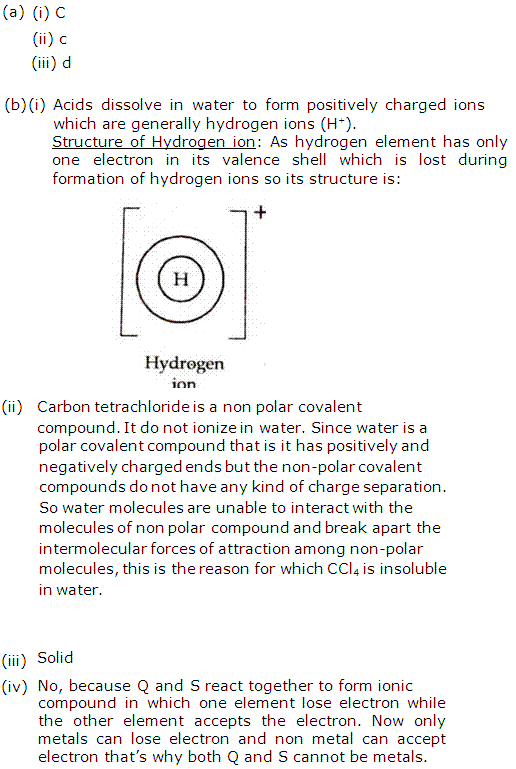
ionises when dissolved in water
Solution 2009-1
(i) solid
(ii) low
Solution 2008-2
(ii) Co-ordinate bond
Solution 2010-1
|
Column A |
Column B |
|
(i) Sodium chloride (ii) Ammonium ion (iii) Carbon tetrachloride |
Ionic bond Covalent and coordinate bond Covalent bond
|
Solution 2011-1
HCl is a covalent compound formed by sharing one electron between chlorine and hydrogen. Because chlorine is more electronegative than hydrogen, the shared pair of electrons shifts towards the chlorine atom. So, a partial negative charge (δ-) develops on chlorine and a partial positive charge (δ+) develops on hydrogen. Hence, the covalent bond is polar in nature.
Solution 2011-2
Chemical Bonding Exercise 47
Solution 2013-1
|
Carbon tetrachloride |
Sodium chloride |
|
It is insoluble in water but dissolves in organic solvents. |
It is soluble in water but insoluble in organic solvents. |
|
It is a non-conductor of electricity due to the absence of ions. |
It does not conduct electricity in the solid state but conducts electricity in the fused or aqueous state. |
Solution 2013-2
Dative or coordinate bond
Solution 2013-3
(c) insoluble in water
Solution 2013-4
(b) Ammonium chloride
Solution 2014-1
b. D
Solution 2014-2
a. B
Solution 2014-3
(a) Ions, Na+ and OH-
(b) Ions, H+ and OH-
(c) Molecules, C12H22O11
Solution 2015-1
(d) Ammonium chloride
Solution 2015-2
|
Atom |
Electronic configuration |
Nearest noble gas |
To attain stable electronic configuration of a nearest noble gas |
|
Carbon |
126C [2,4] |
Neon [2,8] |
Carbon needs four electrons to complete the octet. |
|
Hydrogen |
11H [1] |
Helium [2] |
Hydrogen needs one electron to complete the duplet. |
One atom of carbon shares four electron pairs, one with each of the four atoms of hydrogen.
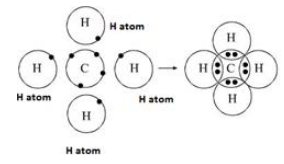
|
|
|
|
Before combination (4 [H] atoms and 1 [C] atom) |
After combination (CH4 - Methane molecule) |
Solution 2015-3
(a) Y + 3e-→ Y3-
(b) 3X +Y2 → X3Y2
Solution 2015-4
(a) Covalent bonding
(b) Reduction
Chemical Bonding Exercise 48
Solution 2016-1
(a) 1. Electrovalent bond
2. Covalent bond
(b) 1. XZ
2. WX
Solution 2016-2
(b) Mainly ions
Solution 2016-3
(a) 17
Solution 2016-4
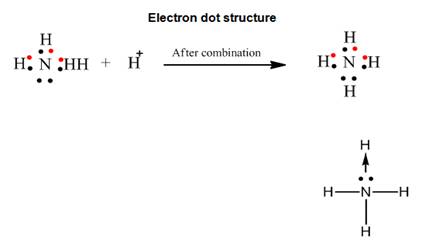
Solution 2017-1
(a) The compound that does not have a lone pair of electrons is carbon tetrachloride.
Solution 2017-2
(c) Always soluble in water
Solution 2017-3
(a) Polar covalent bond
(b) Ionic bond
Solution 2017-4
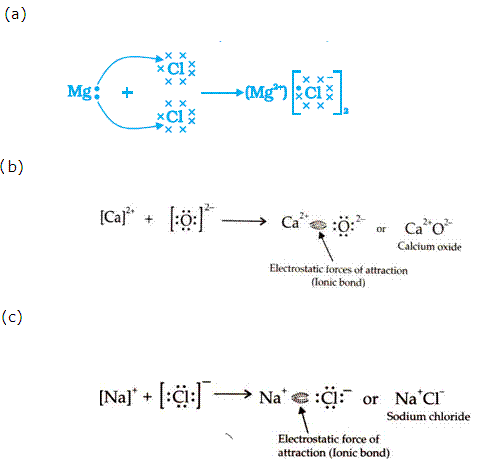
(a)
Formation of carbon tetrachloride
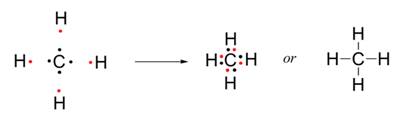
(b)
Electron dot structure of magnesium chloride: