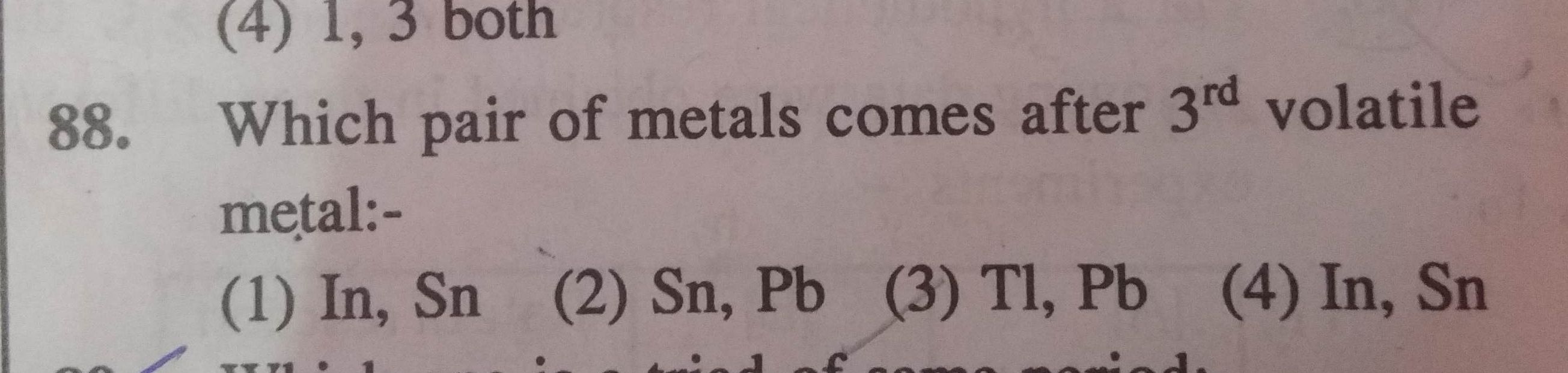NEET Class neet Answered

In case of magnesium, the electron has to be removed from full filled s-orbital. Therefore the first ionization energy of Mg is greater than that of aluminium.
Option 2: Is correct,
Because the size of Mg is smaller than Na and it has greater effective nuclear charge.
In case of Mg has electronic configuration 2p63s2 So it will require less energy to remove an electron from 2p6 in case of sodium.
Therefore, the second ionization potential of sodium is greater than the first ionization energy of magnesium.
option 4: Incorrect. As in Mg the outer electron is unpaired (2p23s1) and less energy will require to remove the electron as compared to aluminium.
So the second ionization potential of Al is greater than that of magnesium.