NEET Class neet Answered
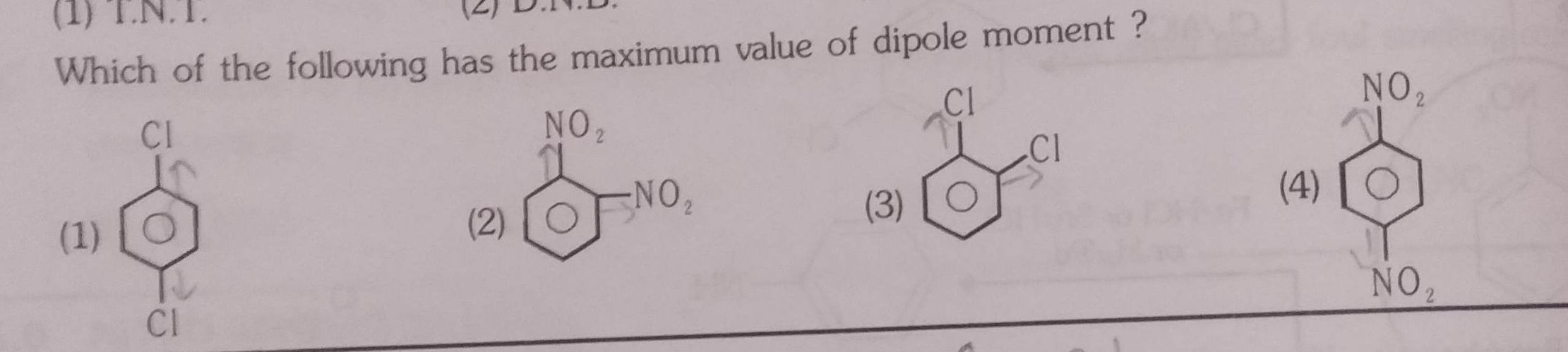
⭕which of the following has the maximum value of dipole moment.plz explain!
Thanks☺️
Asked by jhajuhi19 | 20 Aug, 2020, 10:03: AM
Dipole moment of para dichlorobenzene and para dinitro benzene is zero because both groups are in opposite direction hence they cancel out each other.
In case of ortho isomers nitro group shows more dipole moment than chloro since nitro is electronwithdrawing group.
Hence o-dinitrobenzene shows maximum dipole moment.
Answered by Ramandeep | 21 Aug, 2020, 10:49: AM
NEET neet - Chemistry
Asked by paritoshanjare23 | 26 Jan, 2024, 09:52: PM
NEET neet - Chemistry
Asked by anmol180018 | 13 Jan, 2024, 10:39: PM
NEET neet - Chemistry
Asked by shathwala844 | 27 Jun, 2022, 10:12: AM
NEET neet - Chemistry
Asked by mdaffanmallick28 | 26 May, 2022, 09:52: PM
NEET neet - Chemistry
Asked by mdaffanmallick28 | 26 May, 2022, 09:51: PM
NEET neet - Chemistry
Asked by pallavikumarimddb | 06 May, 2022, 01:54: AM
NEET neet - Chemistry
Asked by neha140602sharma | 26 Apr, 2022, 08:32: AM
NEET neet - Chemistry
Asked by anamika2003.ad | 01 Apr, 2022, 02:12: PM
NEET neet - Chemistry
Asked by yogeshsuthar169 | 14 Nov, 2021, 04:07: PM
NEET neet - Chemistry
Asked by patra04011965 | 30 Jun, 2021, 11:50: AM