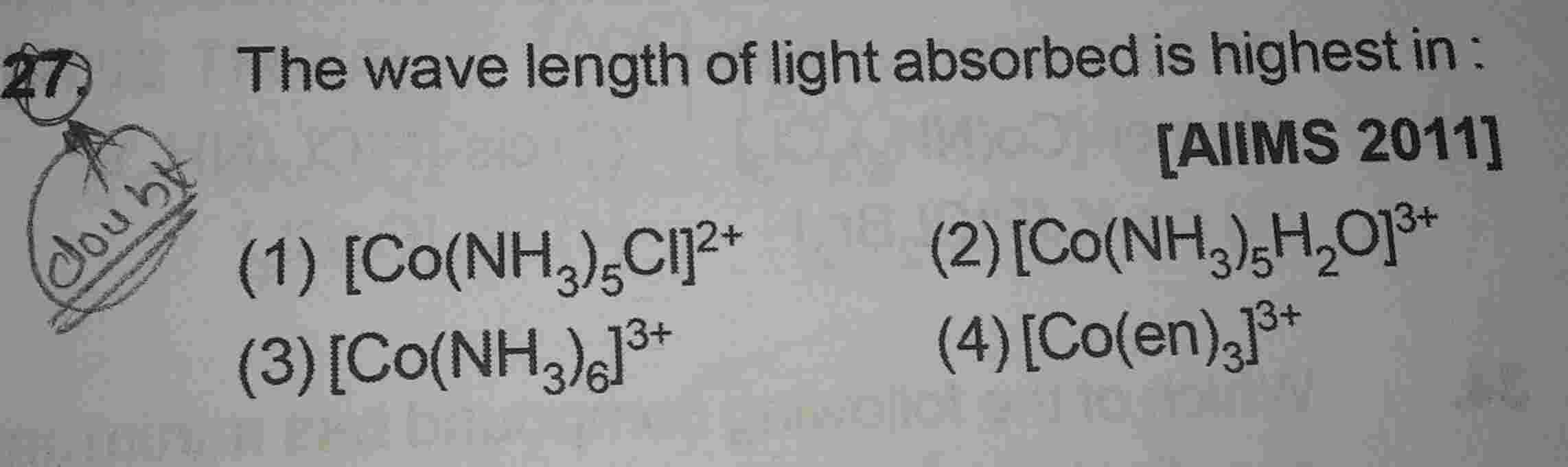NEET Class neet Answered
Factors Affecting Δo:
1. Geometry of the Complex: Δt is approximately 4/9 times of Δo . The lower value of Δt is due to lesser number of ligands in tetrahedral complex.
Given three complxes are octahedral.
2. Oxidation state of metal ion: It is observed that the higher the charge on the central metal atom (or oxidation state), the higher the CFSE.
e.g., Δo for [Fe(CN)6]3- and [Fe (H2O)6]3+ is greater than [Fe (H2O)6]2+
3. Nature of ligand: The value of Δo depends upon the nature of ligands.
Some ligands in spectro chemical series are given below:
I–<Br–<S2– < Cl– < N3–, F– < Urea, OH– < Oxalate, O2– < H2O < NCS– < EDTA < py, NH3 < en =SO3-2 < bipy, phen < NO2– < CH3– < C6H5– < CN– < CO.
For strong field ligands, the order depends on the donor atom and is in the following order:
C-donor > N-donor > O-donor > Halogen donor
In given compounds, [Fe(CN)6]3- and [Fe (H2O)6]3+ , [Fe(CN)6]3- has strong field ligand CN– hence it has greater Δo than [Fe (H2O)6]3+
Complex [Fe(CN)6]3- have greater Δo than rest of given complexes because the charge on central metal ion is +3 and the central metal ion is bonded to a strong ligand CN–