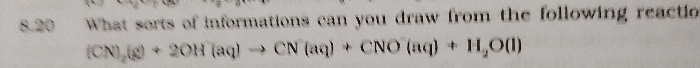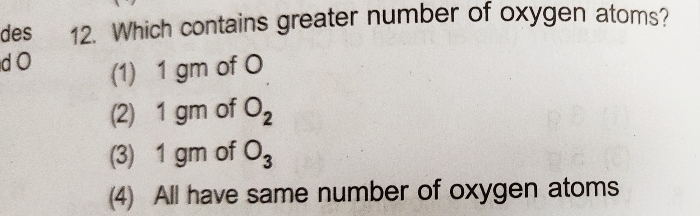NEET Class neet Answered
The work done in adiabatic compression of 2 moles of ideal monatomic gas against constant external pressureof 2 atm starting from initial pressure of 1 atmosphere and initial temperature of 300 Kelvin given R=2 Cal/mole degree
Asked by deepakudgiri29 | 27 Jan, 2019, 11:44: AM
Initial volume Vi is obtained by gas equation : piVi = nRT
Vi = (2×2×4.2×300)/(1.01×105) ≈ 5×10-2 m3
for adiabatic process piViγ = pfVfγ , where γ is ratio of specific heat. For ideal mono-atomic gas γ = 1.67;
hence Vf = Vi (pi / pf )1/γ = 5×10-2 × (1/2)1/1.67 = 3.3×10-2 m3
Workdone for adiabatic process = 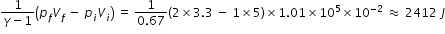
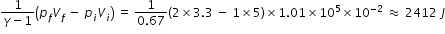
Answered by Thiyagarajan K | 27 Jan, 2019, 15:40: PM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by waghs4548 | 09 Aug, 2024, 20:51: PM
NEET neet - Chemistry
Asked by hdjsiisisii | 03 Aug, 2024, 06:21: AM
NEET neet - Chemistry
Asked by narayandhareppanavar753 | 03 Jul, 2024, 20:03: PM
NEET neet - Chemistry
Asked by khanhazoor446 | 07 Jun, 2024, 12:52: PM
NEET neet - Chemistry
Asked by kishusambhar | 05 Jun, 2024, 11:31: AM
NEET neet - Chemistry
Asked by laxmiprmarlaxmiprmar | 02 Jun, 2024, 01:10: AM
NEET neet - Chemistry
Asked by princejewel712 | 28 May, 2024, 08:26: AM
NEET neet - Chemistry
Asked by priyankasahoo0086 | 22 May, 2024, 06:36: AM
NEET neet - Chemistry
Asked by kanishksingh538 | 22 May, 2024, 00:27: AM