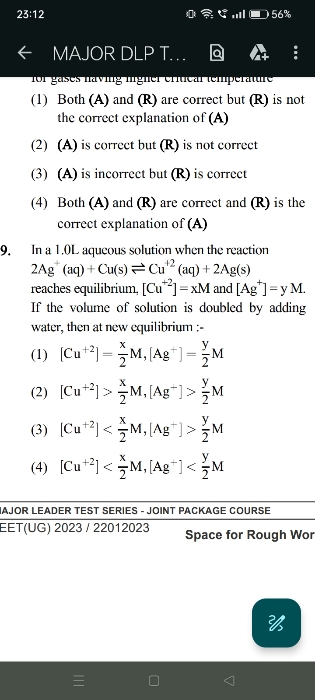
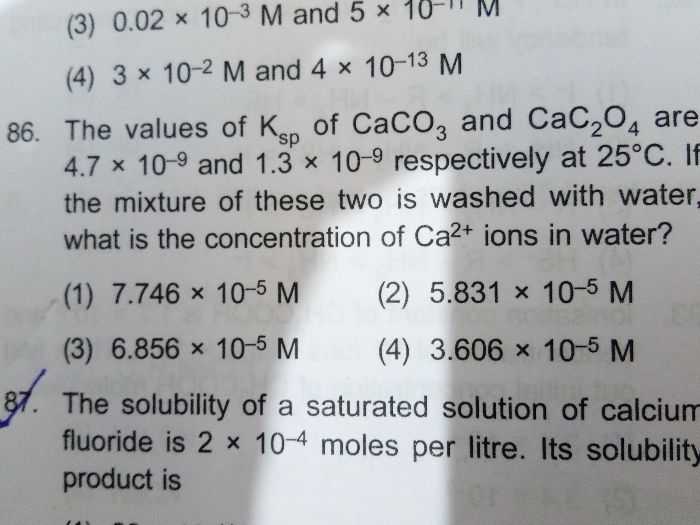
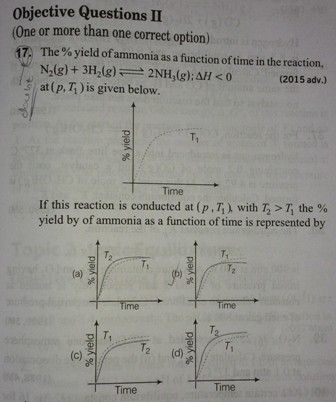
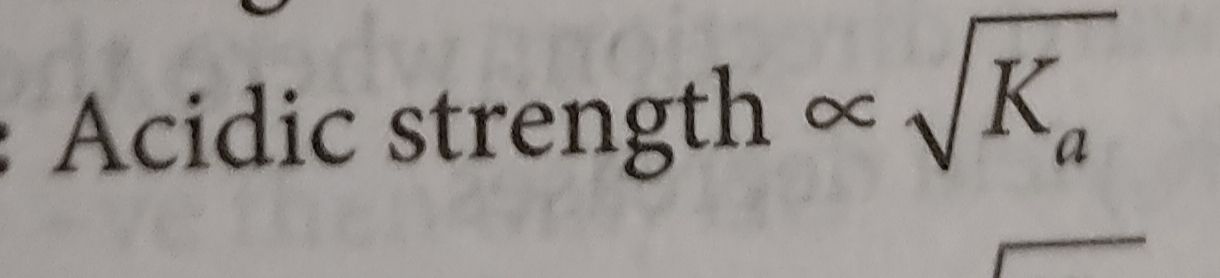NEET Class neet Answered
please answer this
Asked by Prashant DIGHE | 04 Jun, 2020, 10:15: PM
Rate of reaction -
In an exothermic reaction, Rate of reaction is directly proportional to temperature.
T2 > T1
So initially at T2 temperature % yield or formation of NH3 is high. But when equilibrium is acheived it decreases and becomes lesser than at temperature T1 . This trend is illustrated in option (b). So , correct answer is option (b).
Answered by Ravi | 05 Jun, 2020, 10:14: PM
Concept Videos
NEET neet - Chemistry
Asked by ayushkumar111119999 | 03 Mar, 2024, 03:37: PM
NEET neet - Chemistry
Asked by gopad0048 | 20 Feb, 2024, 08:56: AM
NEET neet - Chemistry
Asked by rupalsharma2574 | 28 Jan, 2024, 10:38: PM
NEET neet - Chemistry
Asked by myindiaisbad | 23 Jun, 2022, 09:54: PM
NEET neet - Chemistry
Asked by supriyasonkar28 | 12 Jan, 2022, 05:56: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 13 Jul, 2021, 10:08: AM
NEET neet - Chemistry
Asked by jaashisha65 | 05 Mar, 2021, 12:47: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 13 Jun, 2020, 10:22: PM