NEET Class neet Answered
Please answer this
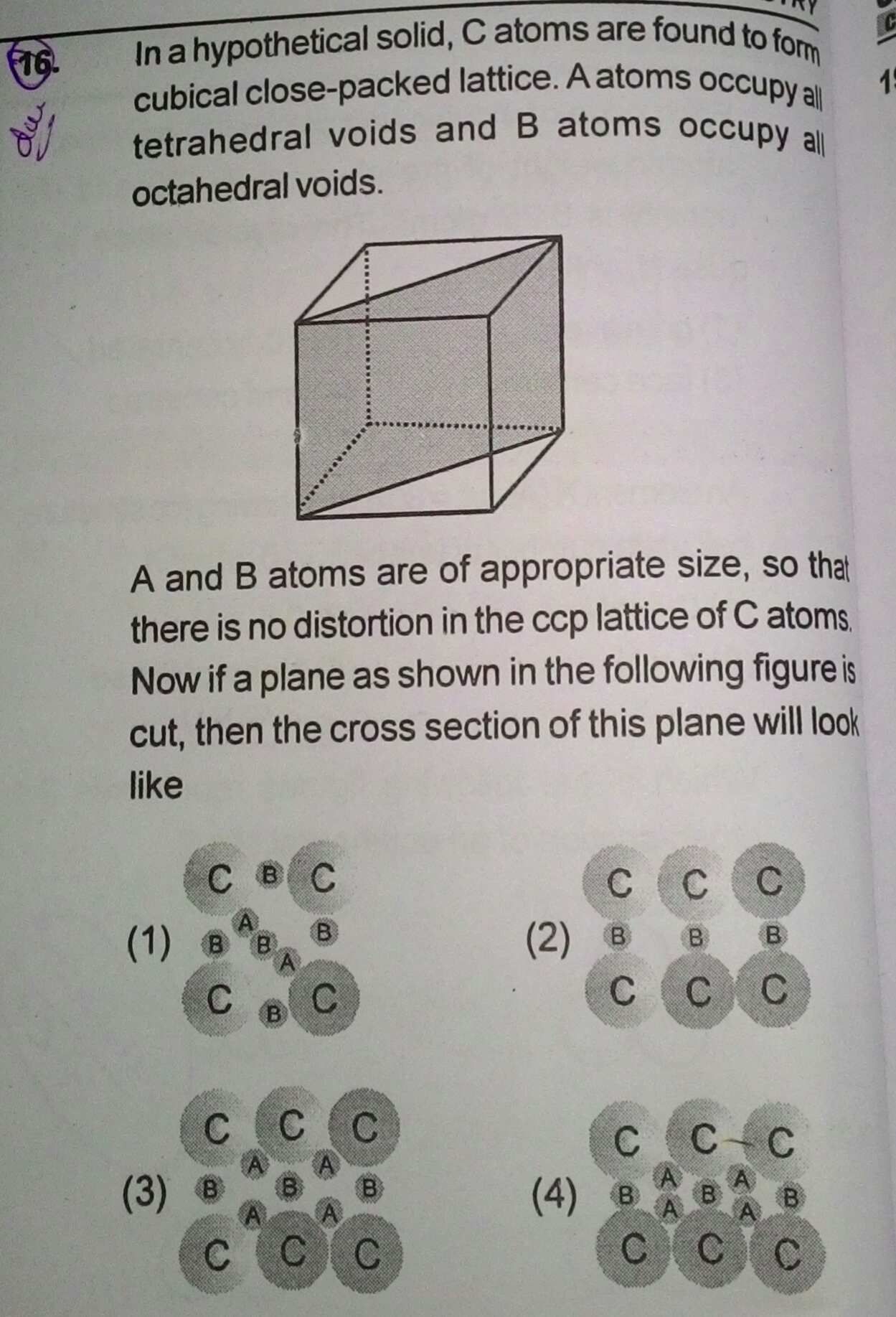
Asked by Prashant DIGHE | 30 Jul, 2019, 07:59: AM
The cross section will look like option (C).
From the given information, the sizes of tetrahedral and octahedral voids, the atoms occupying the voids will not touch each other.
Answered by Varsha | 30 Jul, 2019, 10:08: AM
NEET neet - Chemistry
Asked by Prashant DIGHE | 30 Jul, 2019, 07:59: AM
NEET neet - Chemistry
Asked by shahlaghazal009 | 24 Apr, 2019, 11:28: AM
NEET neet - Chemistry
Asked by inbasri224 | 23 Feb, 2019, 11:46: PM


