NEET Class neet Answered
please answer this
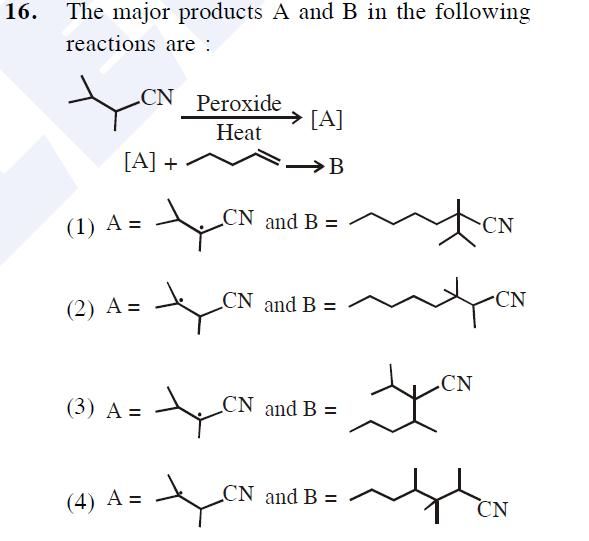
Asked by Prashant DIGHE | 28 Feb, 2020, 10:06: PM
Free radical: An atom or group of atoms having an unpaired electron.
Order of stability of the species: CH3• < 1º < 2º < 3º
Reason for stability order: Larger the number of alkyl groups attached to the carbon atom carrying the odd electron, greater is the delocalization of the odd electron and hence more stable is the free radical.
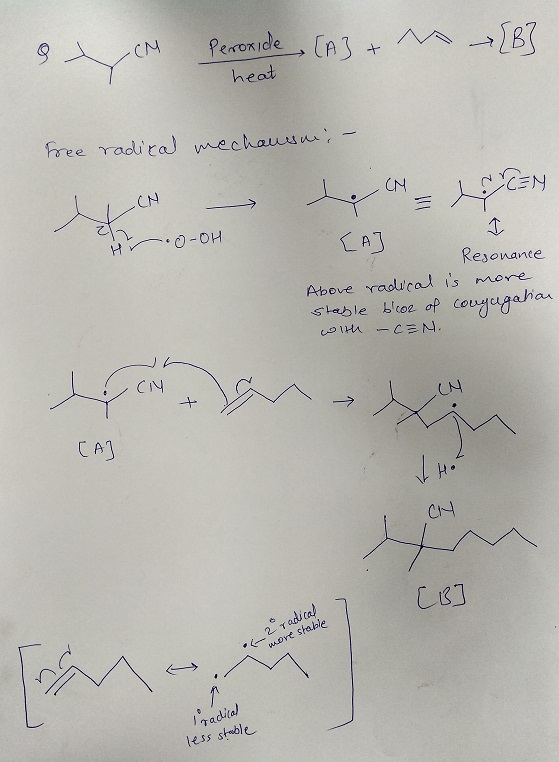
Hence option 1 is the correct answer.
Answered by Ramandeep | 02 Mar, 2020, 12:01: PM
Concept Videos
NEET neet - Chemistry
Asked by Prashant DIGHE | 16 Apr, 2020, 09:27: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 28 Feb, 2020, 10:06: PM






