ICSE Class 10 Answered
One isotope of uranium has a mass number 235 and atomic number 92.
(i) What is the number of electrons in the neutral atom of this isotope?
(ii) What is the number of protons and number of neutrons in its nucleus?
(iii) Do all isotopes have the same number of neutrons?
(iv) What is the number of protons in 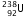 ?
?
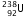 ?
?
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
Given A = 235, Z = 92
Atomic Number Z = number of protons = number of electrons = 92
Therefore, The neutral atom of 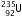 will have 92 electrons.
will have 92 electrons.
(ii) The number of protons = Z = 92
The number of neutrons = A - Z = 235 - 92 = 143.
(iii) No. Isotopes of an element have the same number of protons but different number of neutrons since their mass numbers are different.
(iv) 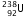 is the isotope of
is the isotope of 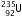 , so it has the same number of protons , i.e. 92.
, so it has the same number of protons , i.e. 92.
Answered by | 04 Jun, 2014, 03:23: PM
ICSE 10 - Physics
Asked by ayush421301 | 29 May, 2019, 11:08: PM
ICSE 10 - Physics
Asked by chumki.banerjee001 | 03 Mar, 2019, 07:10: PM
ICSE 10 - Physics
Asked by pcremyapc33 | 03 Mar, 2019, 12:06: PM
ICSE 10 - Physics
Asked by prodyumna02 | 04 Feb, 2019, 08:28: PM

