ICSE Class 10 Answered
Observation
When kmno4 solution is added to
Ethane
Ethene
Ethyne
Asked by lovemaan5500 | 02 Feb, 2018, 07:47: AM
Observations:
When KMnO4 is added to
1)Ethane:
When KMNO4 is treated with ethane there is no reaction between ethane and KMNO4, the purple colour of KMnO4 has remained same.
2) Ethene:
When KMNO4 is treated with ethene, it oxidizes ethene to give ethane-1,2-diol (ethylene glycol).
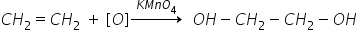
The purple colour of KMNO4 changed to colourless after oxidation.
3) Ethyne:
i) Cold KMNO4 :
Ethyne reacts with cold KMnO4 to form formic acid.
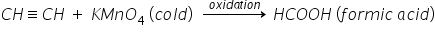
ii) Hot KMNO4 :
Ethyne reacts with hot KMnO4 to form oxalic acid.

Answered by Ramandeep | 02 Feb, 2018, 09:18: AM
ICSE 10 - Chemistry
Asked by casavaish | 19 Jun, 2019, 09:19: PM
ICSE 10 - Chemistry
Asked by DEVIL | 05 Jun, 2019, 04:45: PM
ICSE 10 - Chemistry
Asked by ushanihar12 | 18 Mar, 2019, 04:22: PM
ICSE 10 - Chemistry
Asked by vijaygireesh | 18 Feb, 2019, 03:59: PM
ICSE 10 - Chemistry
Asked by malapandey8278 | 30 Jan, 2019, 01:38: PM
ICSE 10 - Chemistry
Asked by sagarmishra | 19 Mar, 2018, 06:36: PM
ICSE 10 - Chemistry
Asked by kpbhake | 11 Mar, 2018, 12:59: PM
ICSE 10 - Chemistry
Asked by govtsecschoolnayaganv051 | 01 Mar, 2018, 11:49: AM
ICSE 10 - Chemistry
Asked by lovemaan5500 | 02 Feb, 2018, 07:47: AM

 and π bonds
and π bonds