NEET Class neet Answered
how o find dipole moment
Asked by sanj.doha | 25 Nov, 2021, 09:41: AM
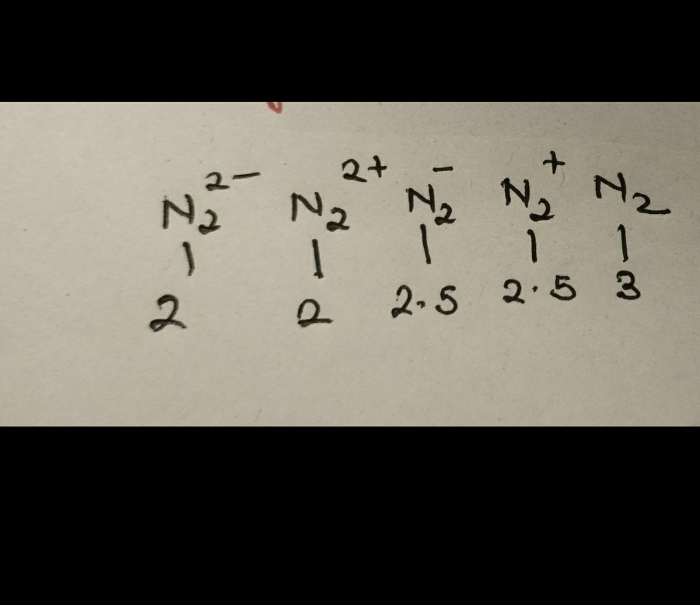
Dipole moment (μ) is defined as the product of the distance of separation between the charges and the magnitude of charge.It is the measure of net molecular polarity.It is the magnitude of the charge Q at either end of the molecular dipole times the distance r between the charges.
μ=Q×r
It tells us about the charge separation in a molecule.The larger the difference in electronegativities of bonded atoms, the larger the dipole moment.For example, the CO2 molecule does not have a dipole moment because it is symmetrical, hence there is no charge separation, while NaCl has the highest dipole moment because it has an ionic bond (i.e. highest charge separation).
It tells us about the charge separation in a molecule.The larger the difference in electronegativities of bonded atoms, the larger the dipole moment.For example, the CO2 molecule does not have a dipole moment because it is symmetrical, hence there is no charge separation, while NaCl has the highest dipole moment because it has an ionic bond (i.e. highest charge separation).
Answered by Ravi | 25 Nov, 2021, 04:20: PM
NEET neet - Chemistry
Asked by anil.kumar.darc | 01 Apr, 2024, 04:19: PM
NEET neet - Chemistry
Asked by kratikarao8412 | 14 Mar, 2024, 08:04: AM
NEET neet - Chemistry
Asked by burriganga777 | 23 Nov, 2023, 06:53: AM
NEET neet - Chemistry
Asked by ranjimasaseendran | 17 Jul, 2023, 01:00: PM
NEET neet - Chemistry
Asked by agarwaldivish01 | 08 Jun, 2022, 03:39: PM
NEET neet - Chemistry
Asked by sanj.doha | 25 Nov, 2021, 09:41: AM
NEET neet - Chemistry
Asked by janhvisingh03022005 | 09 Sep, 2021, 10:32: AM
NEET neet - Chemistry
Asked by vijaya98laxmi | 17 Jan, 2020, 10:24: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 23 Dec, 2019, 09:47: PM