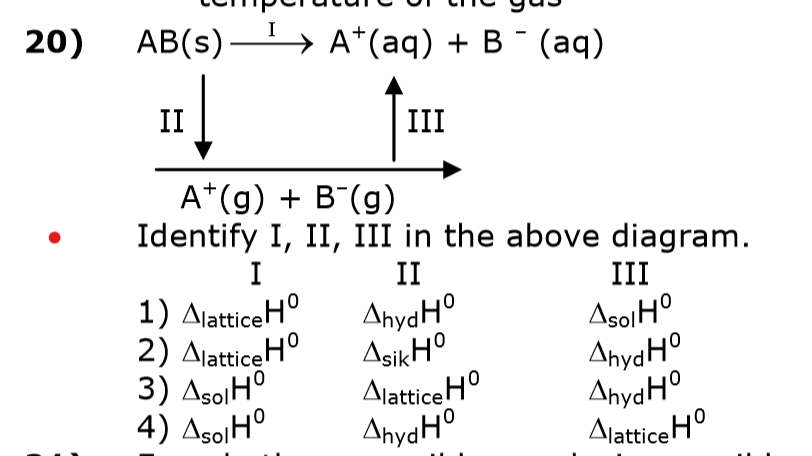
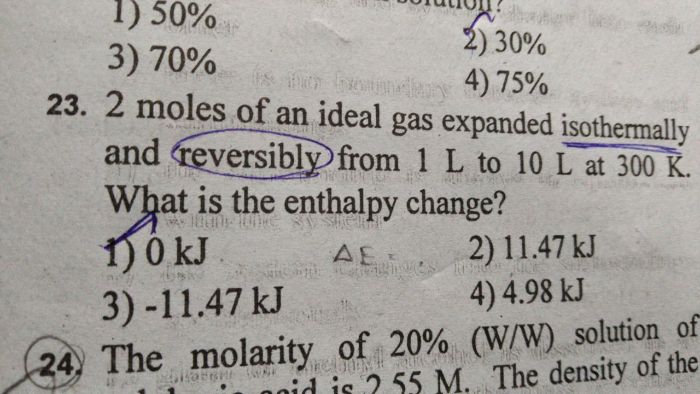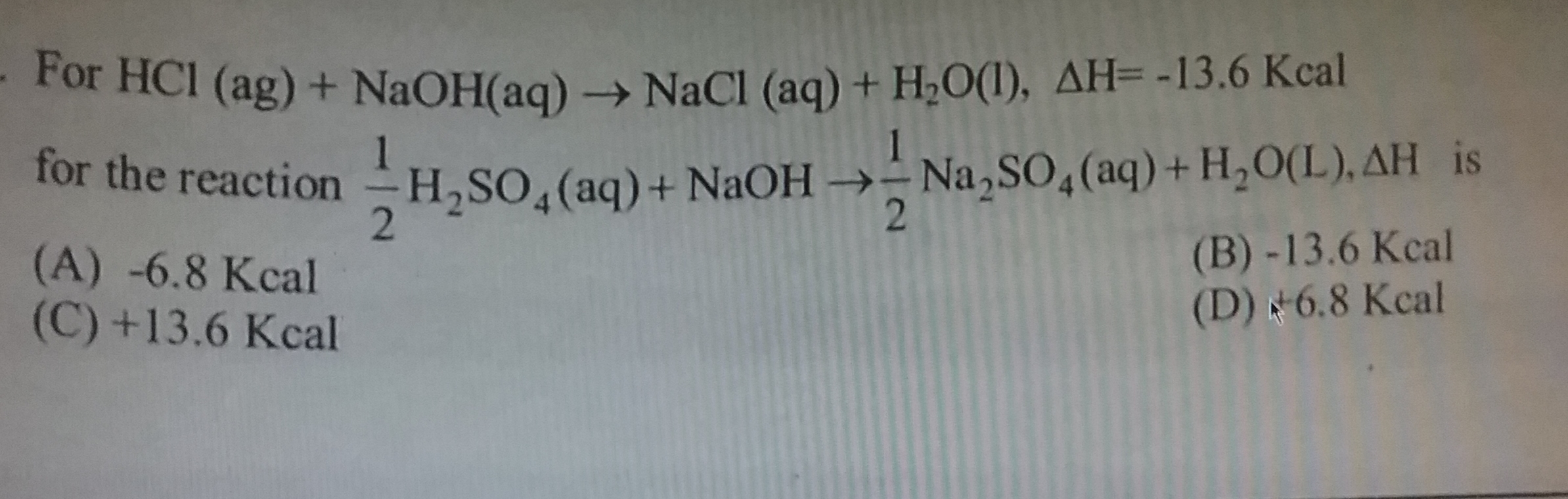Enthalpy
OR
- enthalpy
NEET NEET -
Chemistry
Asked by vasantagomasi23 |
05 April, 2024,
Apr:%i:Friday
NEET NEET -
Chemistry
Asked by shaiksufiyan.klr |
13 January, 2024,
Jan:%i:Saturday
NEET NEET -
Chemistry
Asked by mistymouni2507 |
30 October, 2023,
Oct:%i:Monday
CBSE XI Science -
Chemistry
Asked by advssdrall |
11 January, 2022,
Jan:%i:Tuesday
CBSE XI Science -
Chemistry
Asked by kupadhyay2394 |
03 November, 2020,
Nov:%i:Tuesday
CBSE XI Science -
Chemistry
Asked by Prashant DIGHE |
13 June, 2020,
Jun:%i:Saturday
CBSE XI Science -
Chemistry
Asked by okvgom |
26 April, 2020,
Apr:%i:Sunday
CBSE XI Science -
Chemistry
Asked by varakalasuchi3 |
28 March, 2020,
Mar:%i:Saturday
JEE Main -
Chemistry
Asked by vishakhachandan026 |
15 April, 2019,
Apr:%i:Monday