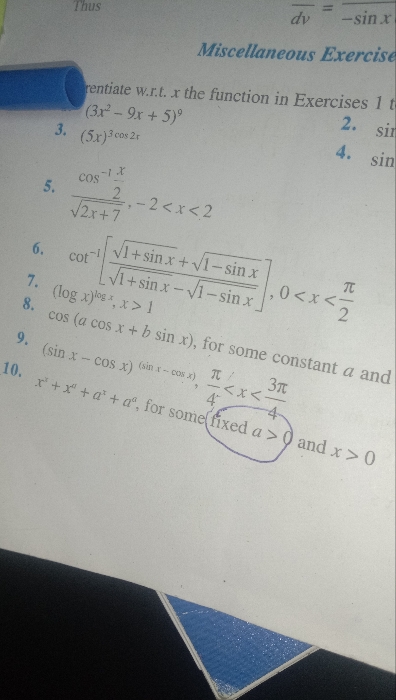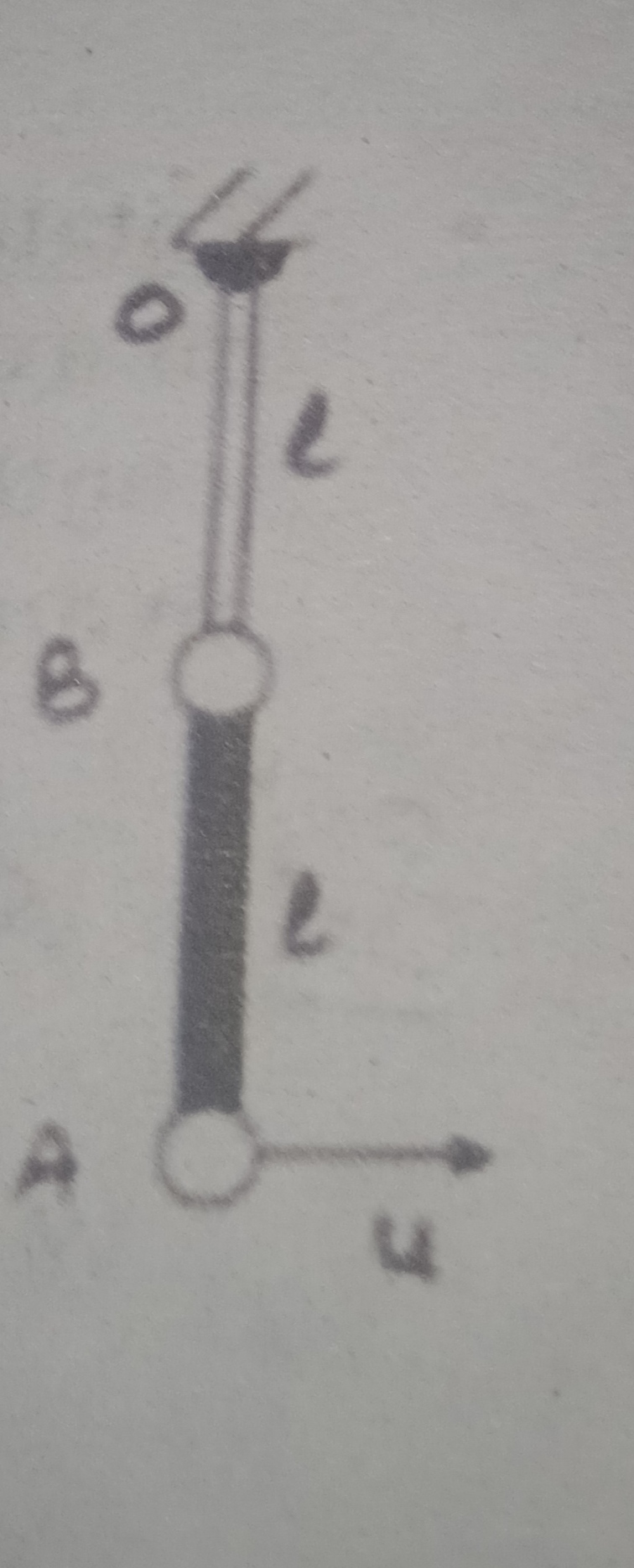CBSE Class 12-science Questions and Answers
CBSE 12-science - Maths
Asked by leelasri11663 | 25 Apr, 2024, 11:34: AM
CBSE 12-science - Maths
Asked by aishaazmata | 24 Apr, 2024, 08:48: PM
CBSE 12-science - Maths
Asked by revengesena | 24 Apr, 2024, 06:08: PM
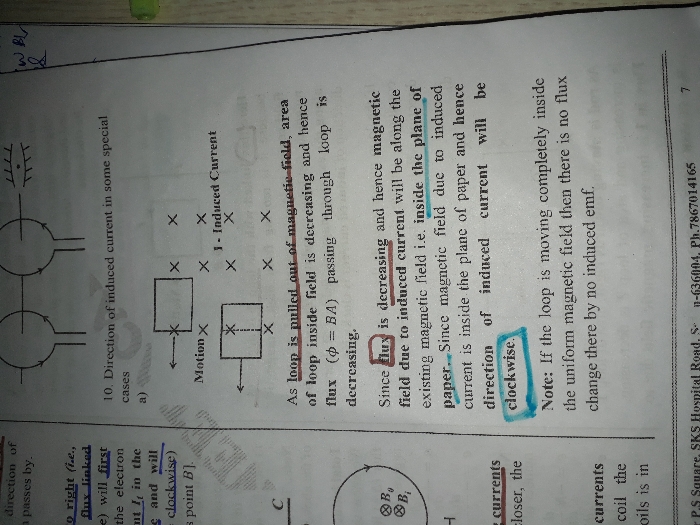
CBSE 12-science - Physics
Asked by panneer1766 | 24 Apr, 2024, 01:52: PM
CBSE 12-science - Physics
Asked by artabandhusahu85 | 24 Apr, 2024, 12:07: PM
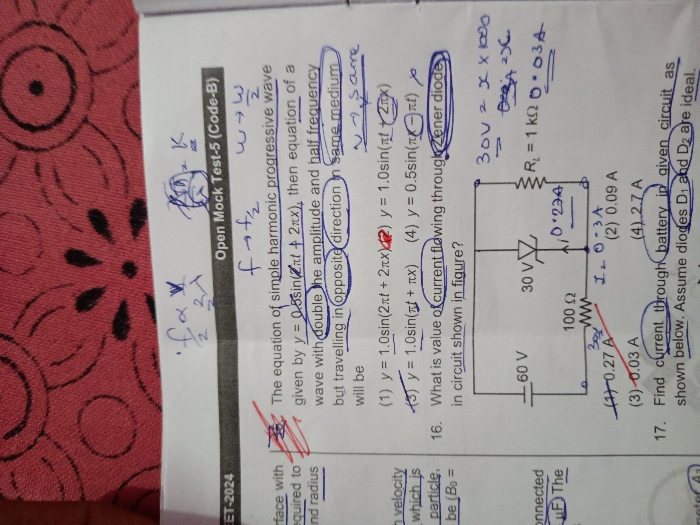
CBSE 12-science - Physics
Asked by niharvijayvargiya5 | 23 Apr, 2024, 06:40: PM
CBSE 12-science - Physics
Asked by kulhariabhijeet | 21 Apr, 2024, 02:39: PM
CBSE 12-science - Physics
Asked by mohapatraswetalina88 | 21 Apr, 2024, 12:18: PM
CBSE 12-science - Physics
Asked by aishaisha091098 | 19 Apr, 2024, 04:54: PM