Three Dimensional Packing Free Doubts and Solutions
CBSE - XII Science - Chemistry - The Solid State
what is solid
CBSE - XII Science - Chemistry - The Solid State
The unit cell of Na is bcc and its density is 0.97. What is the radius of a sodium atom if the molar mass of Na is 23?
CBSE - XII Science - Chemistry - The Solid State
explain please

CBSE - XII Science - Chemistry - The Solid State
pls solve sir
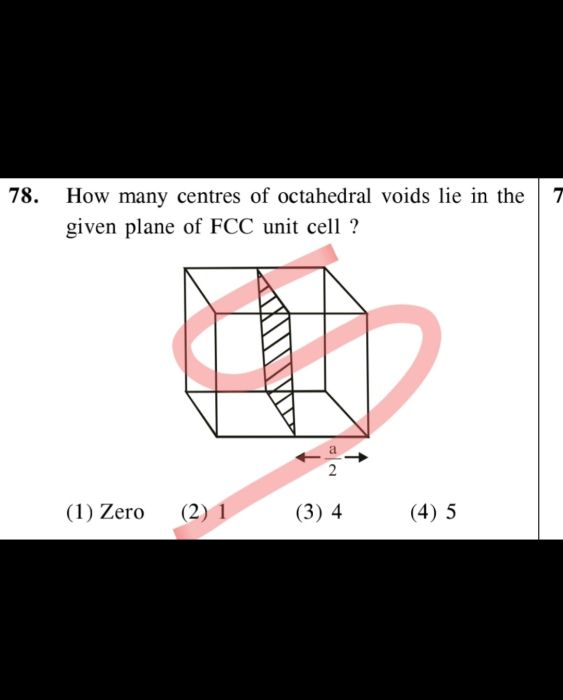
CBSE - XII Science - Chemistry - The Solid State
How many types of 3dimensions are there in a lattice please define.
CBSE - XII Science - Chemistry - The Solid State
sir in types of unit cell we studied it can be of 4 types i.e primitive, face centred, body centred and end centred, but while studing 3D close packing it was told that it can be of 3 types i,e. simple(primitive) cubic unit cell, hcp and ccp. but i am not able to understand that hcp is a type of unit cell or it is one of the 7 crystal system or somthing else?and if it is type of unit cell then why it is not taught under the topic types of unit cell. and if it is a crystal system then what about the rest 6 crystal system, then why rest 6 crystal system does not comes under 3 dimensional close packing?
CBSE - XII Science - Chemistry - The Solid State
In a cubic lattice A atoms having FCC arrangement B atoms are at body centers and C atoms are at alternate edge centers . The formula of the unit cell will be ;
CBSE - XII Science - Chemistry - The Solid State
The number of c4 and c3- axes in a cubic crystal lattice are x and y respectively the value of x+y= a] 2 b] 7
CBSE - XII Science - Chemistry - The Solid State
Hexagonal close packing and cubic close packing
CBSE - XII Science - Chemistry - The Solid State
Analysis shows that nickel oxide has the formula ni 0.98 o1.00. What fractions of nickel exist as ni 2+ and ni 3+ ions?
CBSE - XII Science - Chemistry - The Solid State
What kind of symmetry is exhibited by the macth box?
CBSE - XII Science - Chemistry - The Solid State
STAE DIFFERENCE BETWEEN THE TETRAHEDRAL AND OCTAHEDRAL VOIDS , THEIR LOCATIONS WITH THE HELP OF FIGURE
CBSE - XII Science - Chemistry - The Solid State
Total volume of atom present in a fcc unit cell of a metal is
CBSE - XII Science - Chemistry - The Solid State
What is the formula of a compound in which the element Y forms hcp lattice and atoms of X occupy 2/3rd of tetrahedralvoids .
CBSE - XII Science - Chemistry - The Solid State
Structure of a mixed oxide is CCP. The unit cell is composed of oxide ion. One fourth of the tetrahedral voids are occupied by divalent metal A & the octahedral voids are occupied by monovalent metal B. What will be the formula of the solid?
CBSE - XII Science - Chemistry - The Solid State
the density of Cu is 8.5g/cc.If the radius of Cu atom is 127.8pm.Is the copper unit cell simple cubic,bodycentered or face centered cubic?
CBSE - XII Science - Chemistry - The Solid State
How will you distinguish hexagonal close packing and cubic close packing?
CBSE - XII Science - Chemistry - The Solid State
Explain the cubic close packing.
CBSE - XII Science - Chemistry - The Solid State
Compare the two types of voids formed in cubic close packing.
CBSE - XII Science - Chemistry - The Solid State
Formula of oxide of copper is CuO. If oxide forms the close packing what will be the ratio of octahedral void occupied by copper.
CBSE - XII Science - Chemistry - The Solid State
I want make a model:3d structure of nacl and simple cube tetrahedral and octahedral void how to make 3d model of this I m in confusion that which diagram is best to make
file: brief explanations about type of solid structure details of nacl,cscl,zns,cafe, antifluoride with diagram
so please tell me fast I start making a model and also tell that which materials should be use for making a model
CBSE - XII Science - Chemistry - The Solid State
Why cubic unit cell cannot possess end-centred closed packing?? and what is the highest possible symmetry of this type?
CBSE - XII Science - Chemistry - The Solid State
what is the difference between the hcp and ccp packing ?explain in detail.
CBSE - XII Science - Chemistry - The Solid State
What is three fold axis in case of ABCABC……. or CCP (cubic close packing) in 3 dimentions?
What is six fold axis incase of ABAB…..or hexagonal close packing (hcp) in three dimention?
What is meaning of statement given below-“In hexagonal unit cell three unit cells are shown to give hexagon.”
What is difference between BBC and HCP since both are of ABAB…. Type
Explain in detail with diagram
What is three fold axis in case of ABCABC……. or CCP (cubic close packing) in 3 dimentions?
What is six fold axis incase of ABAB…..or hexagonal close packing (hcp) in three dimention?
What is meaning of statement given below-“In hexagonal unit cell three unit cells are shown to give hexagon.”
What is difference between BBC and HCP since both are of ABAB…. Type
Explain in detail with diagram

