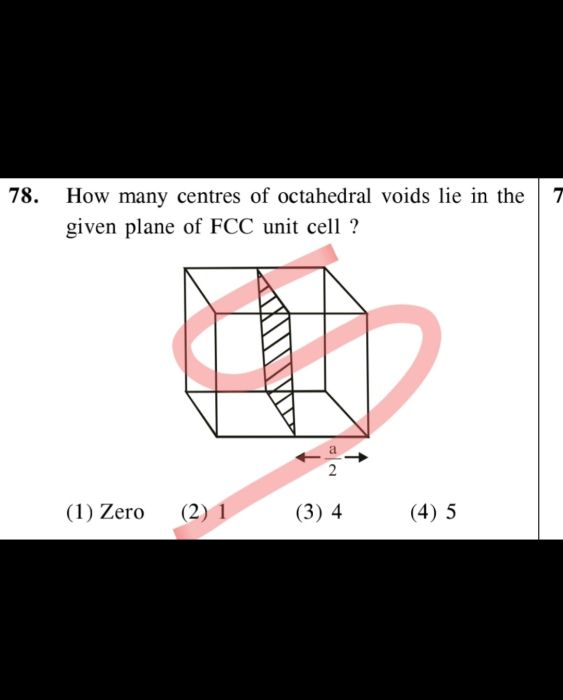
CBSE Class 12-science Answered
If atom A forms the close packing B occupies 1/4 the octahedral void and C occupies the 1/4 the tetrahedral void, find the formula of the compound.
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
Step 1--- If n number of A forms the close packing 2n tetrahedral voids will be formed and n octahedral void will be formed.
Step 2---- If 1/4 the Tetrahedral voids and 1/4 the octahedral voids are occupied then the ratio will be nA : n x 1/4 B : 2n x 1/4 C = A : 1/4 B : 1/2 C, formulas are whole number so multiply by 4
Step 3--- The formula of the compound is A4BC2
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by harshul2019 | 25 May, 2022, 08:52: PM
CBSE 12-science - Chemistry
Asked by arushidabhade | 17 Mar, 2021, 01:24: PM
CBSE 12-science - Chemistry
Asked by kasthurikalvi | 16 Sep, 2020, 03:46: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 01 Jul, 2020, 10:08: PM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 18 May, 2020, 03:00: PM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 10 Sep, 2019, 07:10: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 05 Aug, 2019, 12:08: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 04 Aug, 2019, 09:06: PM
CBSE 12-science - Chemistry
Asked by ranasingh04082002 | 31 Jul, 2019, 02:04: PM
CBSE 12-science - Chemistry
Asked by Saransekar407 | 11 Mar, 2019, 06:51: PM