Properties Of D Block Elements Free Doubts and Solutions
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
Why do transition metals and their compounds show catalytic activities?
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
Why d block elements forms complex compounds?
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
copper nitrate radical test experiment for practical with proper method to write in copy
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
actinide and actinide contraction refers to
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
low reduction potential of iron as well as vanadium is low. why?
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
pls explain
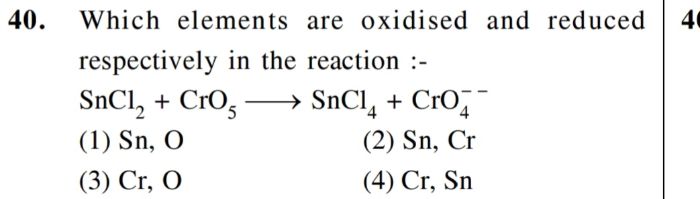
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
please answer this

CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
why Hg exist in liquid state unlike other D block elements?
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
Give solubility order for alkali metal oxides.
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
Give solubility order for i) alkali metal bicarbonates, ii) silver halides.
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
Pl ans fast ..n all the parts pl experts

CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
Why zeff equivalent to screening effect for Fe Co and ni
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
Why zeff greater than screening effect for sc, ti,vn,cr and Mn
CBSE - XII Science - Chemistry - The d-Block and f-Block Elements
Question


