Electrolysis Free Doubts and Solutions
CBSE - XII Science - Chemistry - Electrochemistry
Please answer this

CBSE - XII Science - Chemistry - Electrochemistry
Electrochemistry:- Comment on pH of solution after electrolysis of ZnSO4 in aqueous media by using Zn electrode.
CBSE - XII Science - Chemistry - Electrochemistry
Aluminium is extracted by electrolysis of
CBSE - XII Science - Chemistry - Electrochemistry
Pls explain
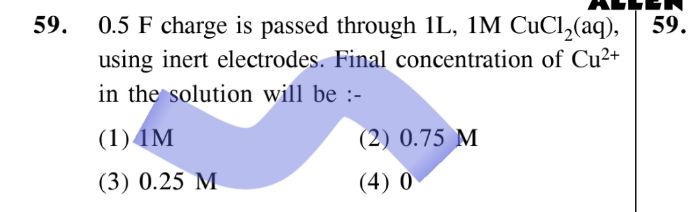
CBSE - XII Science - Chemistry - Electrochemistry
TOTAL VOLUME OF GASES EVOLVED AT STP WHEN 36g OF H2O ARE COMPLETELY ELECTROLYSED BETWEEN PLATINUM ELECTRODES.
CBSE - XII Science - Chemistry - Electrochemistry
What happens to flow of electrons if we introduce two anodes at one end of the cathode
CBSE - XII Science - Chemistry - Electrochemistry
Please answer this question.
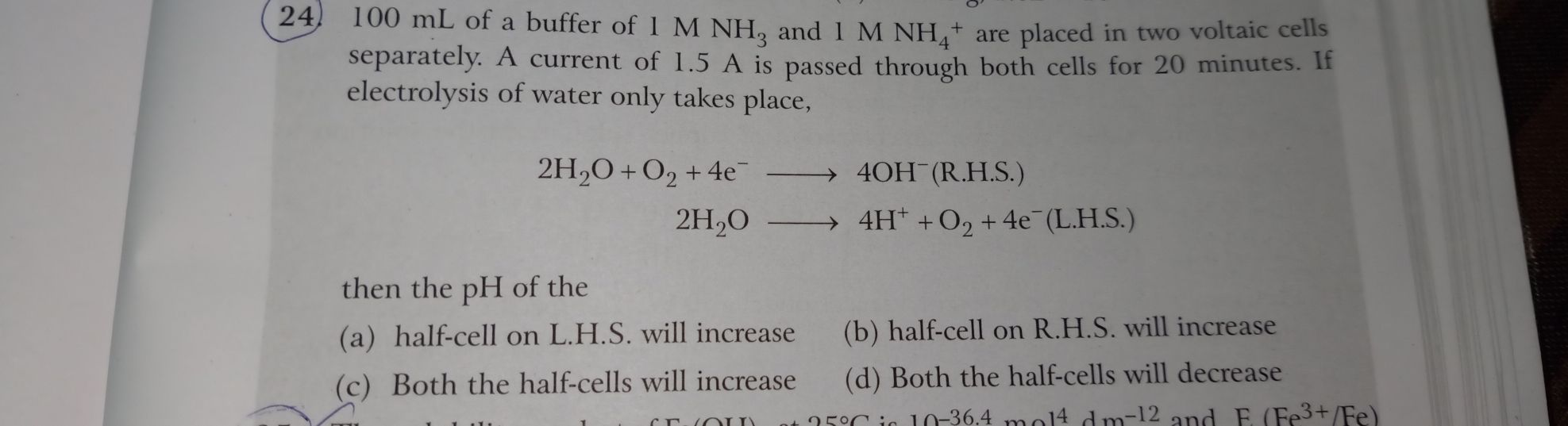
CBSE - XII Science - Chemistry - Electrochemistry
3.66 sum plz

CBSE - XII Science - Chemistry - Electrochemistry
3.71 plz

CBSE - XII Science - Chemistry - Electrochemistry
3.52 numerical plz
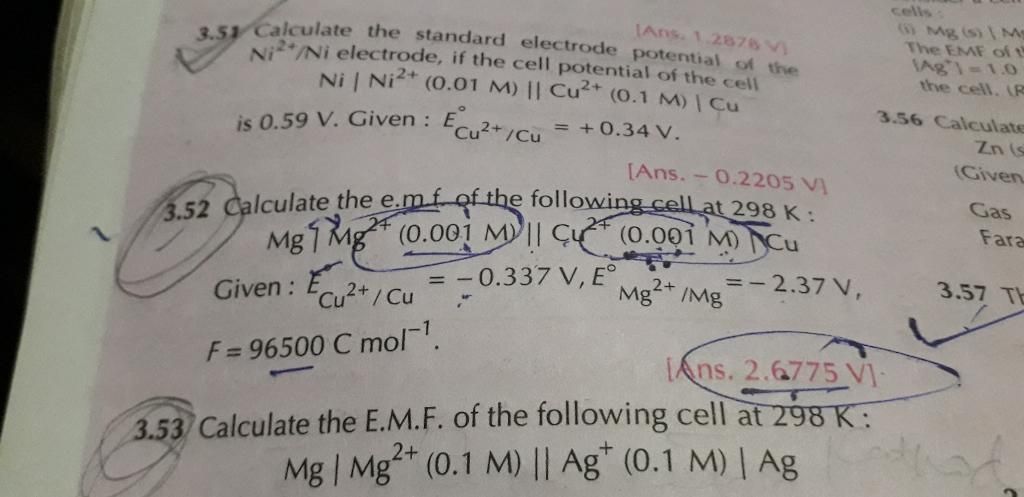
CBSE - XII Science - Chemistry - Electrochemistry
3.57 plz
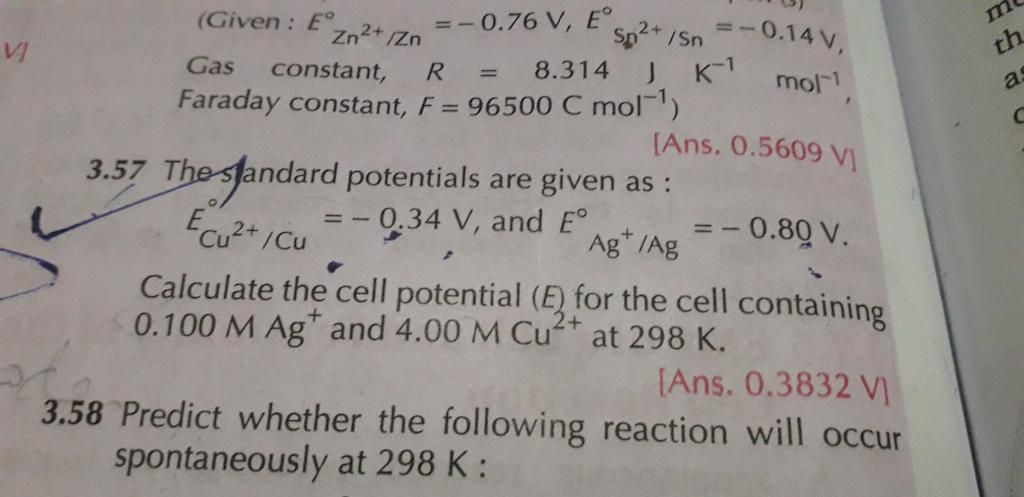
CBSE - XII Science - Chemistry - Electrochemistry
Pl this question as fast as possible
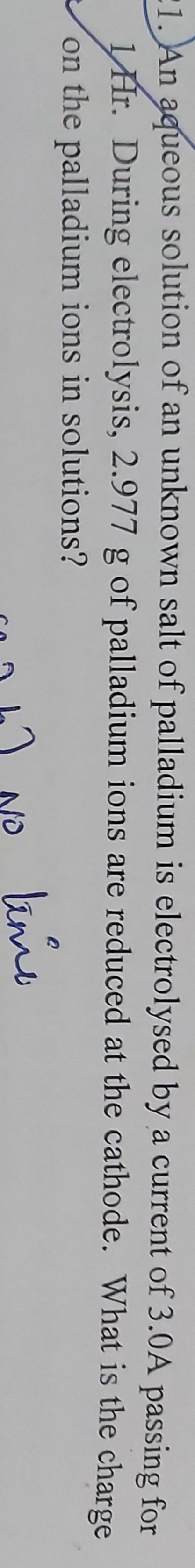
CBSE - XII Science - Chemistry - Electrochemistry
calculate the mass of silver deposited from silver nitrate solution by a current of 2A flowing for 30 minutes
CBSE - XII Science - Chemistry - Electrochemistry
pls explain electroplating
CBSE - XII Science - Chemistry - Electrochemistry
what is molten nacl
CBSE - XII Science - Chemistry - Electrochemistry
Calculate the no.of electrons lost or gained during electrolysis of 1 gm cu2+ ions
CBSE - XII Science - Chemistry - Electrochemistry
what is electrochemistry
CBSE - XII Science - Chemistry - Electrochemistry
Cr metal can be placed out from an acidic solution containing 
Calculate (i) how many grams of Cr will be plated out by 24000 C?
(ii) how long will it take to plate out 1.5g of Cr by using 12.5 A current?
CBSE - XII Science - Chemistry - Electrochemistry
Write the most effective electrolyte on the coagulation of Fe2O3.H2O/Fe3+ sol?
CBSE - XII Science - Chemistry - Electrochemistry
Kindly give detailed answers to the following questions.
24 all parts


CBSE - XII Science - Chemistry - Electrochemistry
Predict the products of electrolysis of an aqueous solution of AgNO3 with silver electrodes. I wrote Ag+,NO3-,H+,OH-.Is it correct?
CBSE - XII Science - Chemistry - Electrochemistry
why gold can be plated better from aucl3 out of aucl3 and naaucl4
CBSE - XII Science - Chemistry - Electrochemistry
how to find log and antilogs of +ve and -ve number
CBSE - XII Science - Chemistry - Electrochemistry
how to plot graph of conductivity vs molar condutivity
CBSE - XII Science - Chemistry - Electrochemistry
100 ml of a neutral solution containing 0.2 g of copper was electrolysed till the whole of copper was deposited. The current strength was maintained at 1.2 amperes and the volume of solution was maintained at 100ml. Assuming 100% efficiency, find out the time taken for deposition of copper. [Atomic weight of copper = 63.58]
CBSE - XII Science - Chemistry - Electrochemistry
Calculate the weight of copper deposited when two faraday of electricity is passed through a cupric salt. (Atomic weight of copper = 63.5)
CBSE - XII Science - Chemistry - Electrochemistry
How many coulombs of electricity are required for the oxidation of 90 g of water?
CBSE - XII Science - Chemistry - Electrochemistry
How much maximum amount of Alumininum metal is deposited at cathode by passing one ampere current for 96500seconds through molten AlCl3?
CBSE - XII Science - Chemistry - Electrochemistry
Three electrolytic cells, A,B,C containing solutions of 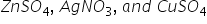 respectively are connected in series. A steady current of 1.5 Amperes is passed through them until 1.45g of Silver was deposited at the cathode of cell B. How long did the current flow? What mass of Copper and Zinc was deposited?
respectively are connected in series. A steady current of 1.5 Amperes is passed through them until 1.45g of Silver was deposited at the cathode of cell B. How long did the current flow? What mass of Copper and Zinc was deposited?
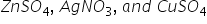 respectively are connected in series. A steady current of 1.5 Amperes is passed through them until 1.45g of Silver was deposited at the cathode of cell B. How long did the current flow? What mass of Copper and Zinc was deposited?
respectively are connected in series. A steady current of 1.5 Amperes is passed through them until 1.45g of Silver was deposited at the cathode of cell B. How long did the current flow? What mass of Copper and Zinc was deposited?
