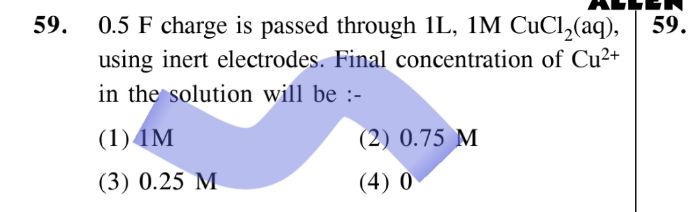
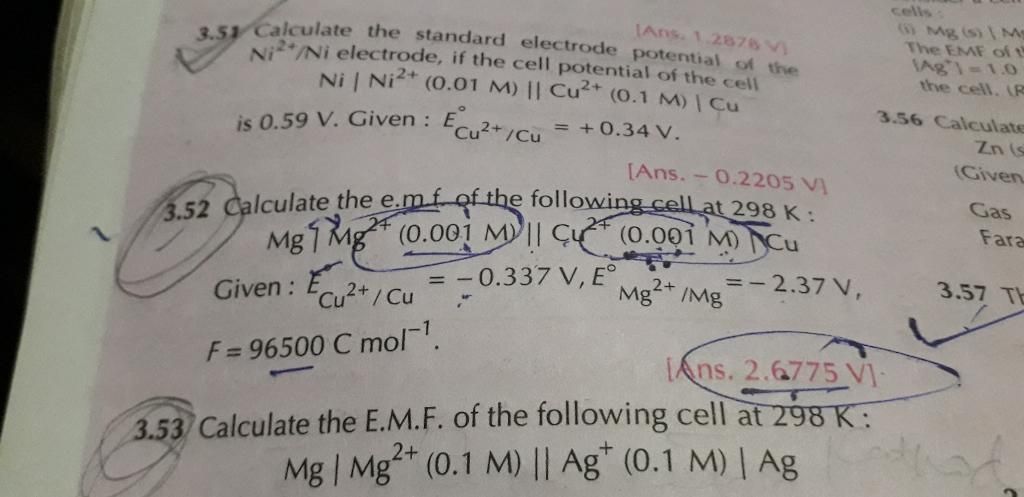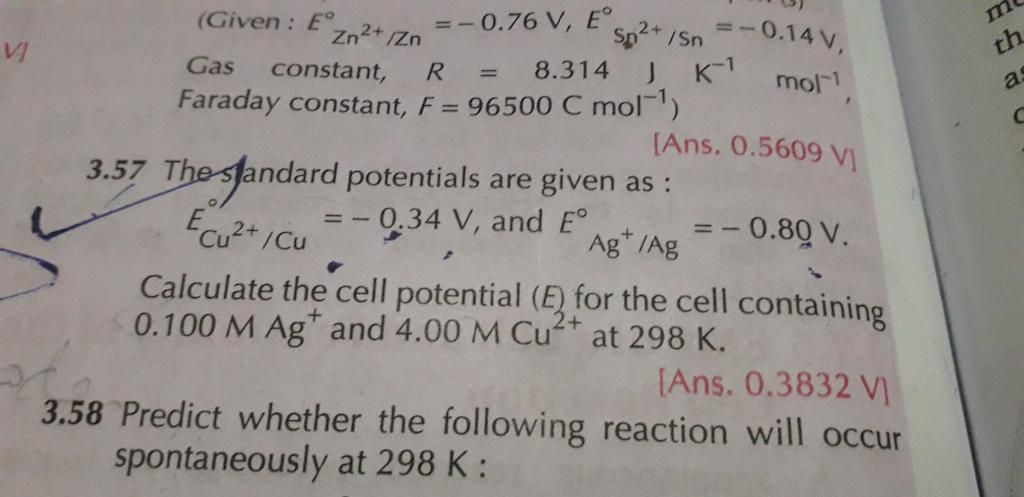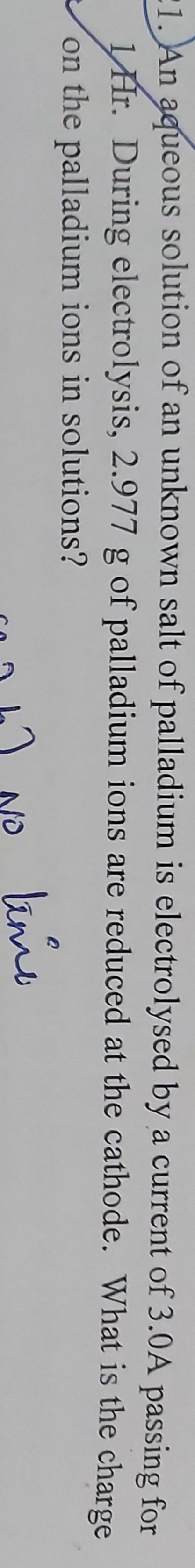CBSE Class 12-science Answered
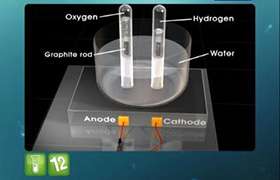
how to Predict the products of electrolysis?
Asked by | 27 Feb, 2008, 05:51: PM
The positive ions will move to the cathode and negative to the anode. The cation which has higher reduction potential will be reduced
in preference will be reduced at cathode and the anion which has higher oxidation potential will be oxidised at anode.
Answered by | 20 Dec, 2017, 03:56: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 05:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 04:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 08:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 09:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:11: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 19 Jul, 2019, 09:49: PM