NEET Class neet Answered
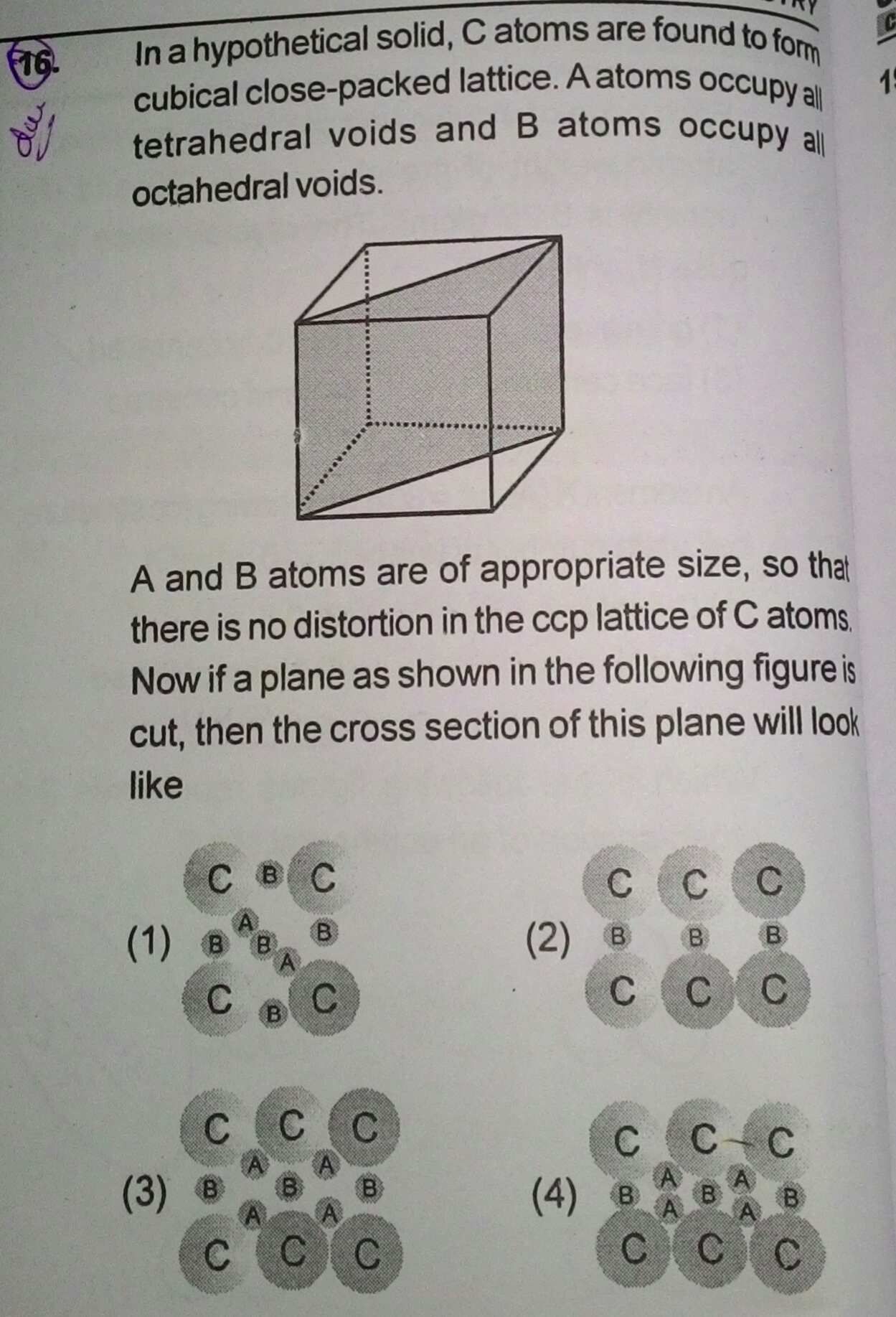
Aroms of element A form CCP lattice and those of element b occupy at centre and body centre the formula of unit cell when all the atoms are removed which are lying on diagonal plane is
a) AB2 b)A2B . c)AB d) A2B3
Asked by inbasri224 | 23 Feb, 2019, 11:46: PM
Atoms of element A forms CCP lattice,
No. of particles per unit cell = 4
Atoms of element B occupy edge center and body center,
No. of particles per unit cell = 3
Therefore the formula is A4B3
When all the atoms from diagonal are removed, that means 2 atoms of A and 2 atoms of B are removed.
So the formula will be A2B.
Answered by Varsha | 26 Feb, 2019, 12:36: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 30 Jul, 2019, 07:59: AM
NEET neet - Chemistry
Asked by shahlaghazal009 | 24 Apr, 2019, 11:28: AM
NEET neet - Chemistry
Asked by inbasri224 | 23 Feb, 2019, 11:46: PM