ICSE Class 10 Answered
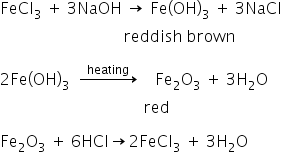
a yellow solution of a salt yields reddish brown precipitate with caustic soda solution .the precipitate does not dissolve in excess of the alkali. the reddish brown precipitate on strong heating leaves behind a red powder, insoluble in water but soluble in dilute HCl . (1)-identify the metal of the salt (2)- write the equation of the reaction involved , assuming the salt to be a sulphate salt.
Asked by kumargangwarn | 18 Jul, 2020, 04:38: PM
Fe(OH)3 Precipitate has reddish brown colour. Salt is haivng yellow colour solution , It is FeCl3 .
Answered by Ravi | 18 Jul, 2020, 10:03: PM

