Equilibrium Free Doubts and Solutions
CBSE - XI Science - Chemistry - Equilibrium
calculate the ph of solution formed by mixing 0.2 M NH4Cl and 0.1 NH3 .(poh of ammonia solution is 4.75)
CBSE - XI Science - Chemistry - Equilibrium
In this question,Nacl precipitate(13.6g) should also be formed along with caso4(20.4g),so the answer should be 13.6+20.4=34g Then why is the answer 20.4g?please explain.

CBSE - XI Science - Chemistry - Equilibrium
Please answer this
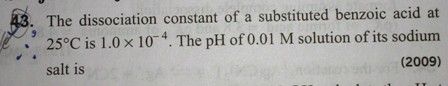
CBSE - XI Science - Chemistry - Equilibrium
please answer this

CBSE - XI Science - Chemistry - Equilibrium
Please answer. It's the same question printed in the next page so I combined it

CBSE - XI Science - Chemistry - Equilibrium
What is the condition for ionisation of hydrogen chloride?
CBSE - XI Science - Chemistry - Equilibrium
For the water gas reaction c(g)+h2o(g)=co(g)+h2(g ) the standard Gibbs free energy for the reaction at 1000k is -8.1 k^3 /mol. Calculate its Equlipriam constant. (R=8.314×10^-3k^3/k/mol).
CBSE - XI Science - Chemistry - Equilibrium
Q) At 540K, 0.10 moles of PCL5 are heated in a 8 litre flask. The pressure of the equilibrium mixture is found to be 1.0 atm. Calculate Kp and Kc for the reaction.
CBSE - XI Science - Chemistry - Equilibrium
Q) The equilibrium composition for the reaction is -




