CBSE Class 11-science Answered
For the water gas reaction c(g)+h2o(g)=co(g)+h2(g ) the standard Gibbs free energy for the reaction at 1000k is -8.1 k^3 /mol. Calculate its Equlipriam constant. (R=8.314×10^-3k^3/k/mol).
Asked by Venkatasubrmanian | 27 Mar, 2019, 10:30: AM
Given:
Gibbs free energy ΔG0 = −8.1 kJ
= 8100 J
Temperature, T = 1000 K
Gas constant, R = 8.314 J/mol K
We know,
Equilibrium constant kp =
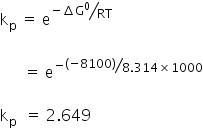
Answered by Varsha | 27 Mar, 2019, 01:04: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by visank90 | 24 Nov, 2023, 10:45: AM
CBSE 11-science - Chemistry
Asked by gouravvv641 | 16 Aug, 2022, 09:25: PM
CBSE 11-science - Chemistry
Asked by mangalchandrj79 | 21 May, 2022, 04:38: PM
CBSE 11-science - Chemistry
Asked by sarojlaxmiacharjya | 03 Jan, 2022, 08:50: PM
CBSE 11-science - Chemistry
Asked by cjam41665 | 09 Oct, 2021, 11:11: PM
CBSE 11-science - Chemistry
Asked by rishika62124 | 03 Mar, 2021, 05:02: AM
CBSE 11-science - Chemistry
Asked by jyotijhajharia39 | 06 Jan, 2021, 11:41: PM
CBSE 11-science - Chemistry
Asked by nsaikumar33 | 15 Aug, 2020, 11:50: AM
CBSE 11-science - Chemistry
Asked by swati2678 | 10 Aug, 2020, 01:58: PM
CBSE 11-science - Chemistry
Asked by veenatripathi | 28 May, 2020, 09:03: AM










