CBSE Class 11-science Answered
Q) At 540K, 0.10 moles of PCL5 are heated in a 8 litre flask. The pressure of the equilibrium mixture is found to be 1.0 atm. Calculate Kp and Kc for the reaction.
Asked by Anish | 07 Feb, 2019, 05:20: PM
The reaction is
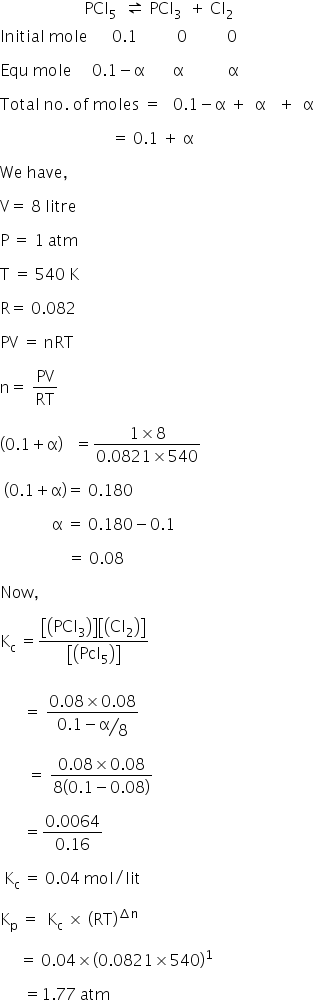
Equilibrium constant Kp 1.77
Kc = 0.04
Answered by Varsha | 07 Feb, 2019, 07:09: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by visank90 | 24 Nov, 2023, 10:45: AM
CBSE 11-science - Chemistry
Asked by gouravvv641 | 16 Aug, 2022, 09:25: PM
CBSE 11-science - Chemistry
Asked by mangalchandrj79 | 21 May, 2022, 04:38: PM
CBSE 11-science - Chemistry
Asked by sarojlaxmiacharjya | 03 Jan, 2022, 08:50: PM
CBSE 11-science - Chemistry
Asked by cjam41665 | 09 Oct, 2021, 11:11: PM
CBSE 11-science - Chemistry
Asked by rishika62124 | 03 Mar, 2021, 05:02: AM
CBSE 11-science - Chemistry
Asked by jyotijhajharia39 | 06 Jan, 2021, 11:41: PM
CBSE 11-science - Chemistry
Asked by nsaikumar33 | 15 Aug, 2020, 11:50: AM
CBSE 11-science - Chemistry
Asked by swati2678 | 10 Aug, 2020, 01:58: PM
CBSE 11-science - Chemistry
Asked by veenatripathi | 28 May, 2020, 09:03: AM










