Physical And Chemical Changes Worksheet
Objective Type Questions
- Select the odd one out and give reason for the same.
(a)Cake baking
(b)Digestion of food
(c)Wood cutting
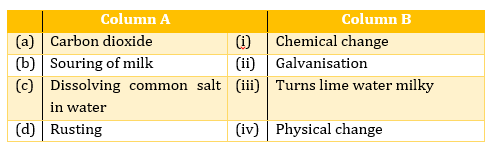
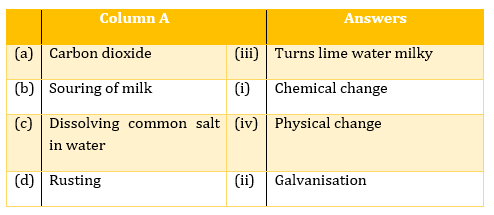
(d)Candle burning - Match the terms in column A with appropriate terms in column B.
- State whether the following statements are true or false. In case of false statement, write the correct one.
(i)Photosynthesis is a physical change.
(ii)Tearing of paper is an irreversible process, hence it is a chemical change.
Multiple Choice Questions - Renu bought few iron nails for the house renovation. Few of those were used for window hanging while other were drilled in wall for hanging murals. Which of them would get rust easily?
(a) Window
(b) Wall
(c) Both of above
(d) None of above - Doctor explained Abhishek how eating an egg daily would help him to recover from protein deficiency. Breakdown of food into simpler substances is:
(a) a physical change
(b) a chemical change
(c) physical as well as chemical change
(d) None of the above - Kiran burnt tip of a magnesium ribbon. It burnt with a bright white flame and some gases before it turned to ashes.
(i) Magnesium melts, get vapourised and again solidifies into ash.
(ii) Magnesium changes its colour and appearance to form ash
(iii) Magnesium metal reacts with oxygen in air to form new substance.
Which of the above statements about this process is/are correct?
(a) Only (i)
(b) (ii) and (iii)
(c) Only (iii)
(d) All of three - Sunil’s uncle gifted him a box full of mangoes while leaving from native and told him to keep a check on them daily and finish. Next day, Sunil saw that fresh mangoes had ripened, and two of the ripe mangoes had rotted. What kind of change occurred within the fresh mangoes?
(a) Physical change as the appearance of mangoes changed
(b) Chemical change as the smell of mango changed.
(c) Physical change as the colour of mangoes changed.
(d) Chemical change as ripening process cannot be reversed. - Rita dipped an iron nail in copper sulphate solution then after some time, the colour of the solution changes from blue to pale green and iron nail gets covered by red coloured deposit. Change of the colour can indicate both, a chemical as well as a physical change.
What type of change does this experiment signify?
(i) Chemical change because of red coloured deposit on iron nail
(ii) Physical change because colour of the solution changes
(a) Only (i)
(b) Only (ii)
(c) Both (i) and (ii)
(d) None of the above - Prachi mixes flour, milk, eggs and water to create a batter for baking cake. The steps in the process are:
Step 1: Mix flour, water, eggs, sugar and milk in a bowl.
Step 2: Place the batter in a baking tray
Step 3: Bake in the oven.
Which of the following is the correct option?
(a)Step 1: Physical change, Step 2: Chemical change, Step 3: Chemical change
(b)Step 1: Physical change. Step 2: Physical change, Step 3: Chemical change
(c)Step 1: Chemical change, Step 2: Physical change, Step 3: Physical change
(d)Step 1: Chemical change, Step 2: Physical change, Step 3: Chemical change
Subjective Questions - Justify: Candle burning is physical as well as chemical change.
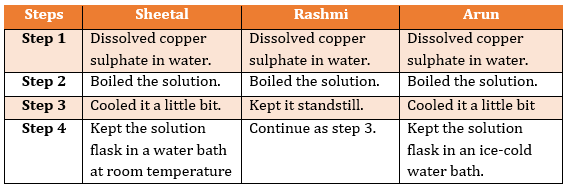
- Teacher asked Sheetal, Rashmi and Arun to prepare pure crystals of copper sulphate from its solution. All three of them procured the required material and followed the steps as shown below:
Whose flask would the first crystals of copper sulphate appear in and why?
(a)Sheetal
(b)Rashmi
(c)Arun
(d)All of three - Distinguish between physical and chemical properties?
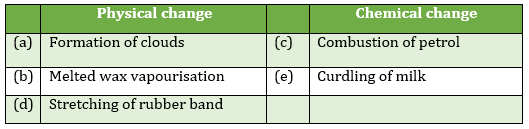
- Classify below events into physical and chemical changes.
(a) Formation of clouds
(b) Melted wax vapourisation
(c) Combustion of petrol
(d) Stretching of rubber band
(e) Curdling of milk - Ridhan left out a block of ice cream, 4 cm in length, in a bowl for some time. It melted into liquid. He then kept the bowl in the freezer. Now, the ice cream formed has the diameter of the bowl. What kind of change occurs in the ice cream and why?
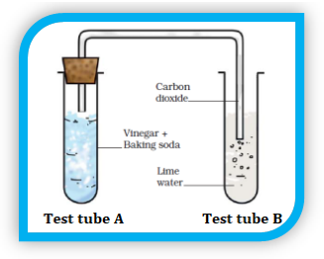
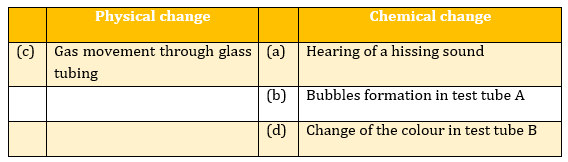
- Akshata added a bit of baking soda to vinegar in a test-tube. She heard a hissing sound and saw several bubbles in the test tube. She closed the test tube with a cork that is attached to another test tube containing lime water by a glass tubing. The limewater slowly turns milky in the process. This is shown in the following experimental setup.
Below mentioned are the observations:
(a) Hearing of a hissing sound
(b) Bubbles formation in test tube A
(c) Gas movement through glass tubing
(d) Change of the colour in test tube B
Categorise these observations into physical changes and chemical changes.
Case-Based Questions - Read the passage and answer the following questions (a), (b) and (c):
It is known that ships are made of iron, and a part of them is submerged under water. Also, water drops cling to the ship's outer surface above water. Additionally, sea water contains many salts. As a result of the salt water, rust forms more quickly. Despite being painted, ships suffer a lot from rusting.
(a) Express rusting in the form of an equation.
(b) Which essential environmental elements assist rusting?
(c) Suggest any two ways to prevent rusting. - Read the passage and answer the following questions (a), (b) and (c):
In Class VI you have learnt that salt can be obtained by the evaporation of sea water. The salt obtained in this manner is not pure and the shape of its crystals cannot be seen clearly. However, large crystals of pure substances can be formed from their solutions.
(a) Define crystallisation.
(b) Select which of the following substances when kept overnight can be recovered from water without any loss? If ‘Yes’, then how and if ‘No’ then why?
(i) Common salt dissolved in water
(ii) Iron nail kept in water
(iii) Sugar dissolved in water
(iv) Copper plate kept in water
(c) Give two applications of crystallisation.
Assertion-Reasoning Questions
Below questions consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below: - Assertion (A): Breaking of a glass is a physical change.
Reason (R): Breaking is an irreversible change. - Assertion (A): Candle burning is a physical change.
Reason (R): Change in the physical properties such as size, shape, state is termed as physical change. - Assertion (A): Ozone protects us from harmful UV rays coming from the Sun.
Reason (R): Oxygen reacts with UV rays to form ozone molecule.
Explore more Science Sample papers and Solutions
-
Sample Papers for CBSE Class 7 Science Term 1 #1
-
Sample Papers for CBSE Class 7 Science Term 2 #1
-
Nutrition in Plants Worksheet
- Nutrition in Animals Worksheets
- Heat Worksheet
- Acids, Bases And Salts Worksheet
- Respiration in Organisms Worksheet
- Transportation In Animals And Plants Worksheet
- Reproduction in Plants Worksheet
- Motion And Time Worksheet
- Electric Current And Its Effects Worksheet
- Light Worksheet
- Forests: Our Lifeline Worksheet
- Wastewater Story Worksheet
- Competency Based Questions for CBSE Class 7 Science
- Sample Papers for CBSE Class 7 Science Term 1 #2
- Sample Papers for CBSE Class 7 Science Term 2 #2
Key Features of Study Materials of CBSE Class 7 Science:
- Easiest and most comprehensive study materials
- Designed by subject matter experts
- Revised according to the latest CBSE syllabus
- Helpful for quick revision
- ‘Ask a Doubt’ facility
- Significant improvement in marks