ICSE Class 10 Answered
Uranium nucleus 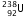 undergoes several disintegrations and ultimately decays to lead nucleus
undergoes several disintegrations and ultimately decays to lead nucleus 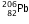 . How many alpha and beta particles are emitted?
. How many alpha and beta particles are emitted?
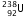 undergoes several disintegrations and ultimately decays to lead nucleus
undergoes several disintegrations and ultimately decays to lead nucleus 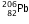 . How many alpha and beta particles are emitted?
. How many alpha and beta particles are emitted?
Asked by Topperlearning User | 21 May, 2014, 04:21: PM
When 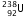 decays to
decays to 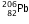 , the mass number decreases from 238 to 206 i.e., it decreases by 32. Since with the emission of one alpha particle, mass number decreases by 4, so total number of alpha particles emitted will be 32/4 = 8.
, the mass number decreases from 238 to 206 i.e., it decreases by 32. Since with the emission of one alpha particle, mass number decreases by 4, so total number of alpha particles emitted will be 32/4 = 8.
In the decay of 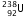 to
to 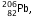 the atomic number has decreased by 10. But due to emission of 8 alpha-particles, the atomic number would have decreased by 2 x 8 = 16. Thus there is an increase in atomic number by 6, hence 6 beta particles will be emitted (because in emission of one beta particle, atomic number increases by 1.)
the atomic number has decreased by 10. But due to emission of 8 alpha-particles, the atomic number would have decreased by 2 x 8 = 16. Thus there is an increase in atomic number by 6, hence 6 beta particles will be emitted (because in emission of one beta particle, atomic number increases by 1.)
Thus 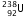 undergoes several disintegrations and ultimately decays to
undergoes several disintegrations and ultimately decays to 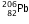 with the emission of 8
with the emission of 8 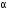 -particles and 6
-particles and 6 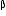 -particles.
-particles.
Answered by | 21 May, 2014, 06:21: PM
ICSE 10 - Physics
Asked by ayush421301 | 29 May, 2019, 11:08: PM
ICSE 10 - Physics
Asked by chumki.banerjee001 | 03 Mar, 2019, 07:10: PM
ICSE 10 - Physics
Asked by pcremyapc33 | 03 Mar, 2019, 12:06: PM
ICSE 10 - Physics
Asked by prodyumna02 | 04 Feb, 2019, 08:28: PM

