JEE Class main Answered
Solve

Asked by sarveshvibrantacademy | 12 May, 2019, 13:47: PM
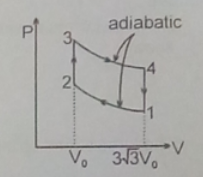
(A) internal energy decreases in the path 3-4
True.
By first law of thermodynamics, dq = du + dw ....................(1)
where dq is the heat energy input , du is change in internal energy and dw is the workdone by the gas.
for adiabatic process, dq = 0, workdone by the gas dw= ∫pdv is positive,
hence du = -dw or internal energy is decreasing.
(B) no work is done during the path 2-3, because volume is constant and change in volume dv = 0
(C) during the path 4-1, volume is constnat.
For ideal gas when volume is constant pressure P is directly proportional to Temperature T.
Since pressure decreasing in the path 4-1, Temperature also devreasing.
(D) during the path 1-2 , workdone dw= ∫pdv , change of volume is negative.
Hence workdone by the gas is negative. Hence work is done on the gas
Answered by Thiyagarajan K | 12 May, 2019, 15:26: PM
Application Videos
Concept Videos
JEE main - Physics
Asked by sumalathamadarapu9 | 23 Oct, 2024, 22:06: PM
JEE main - Physics
Asked by py309649 | 13 Oct, 2024, 13:39: PM
JEE main - Physics
Asked by coolskrish | 13 Oct, 2024, 12:50: PM
JEE main - Physics
Asked by midnightmoon3355 | 09 Oct, 2024, 09:09: AM
JEE main - Physics
Asked by rambabunaidu4455 | 03 Oct, 2024, 16:03: PM
JEE main - Physics
Asked by ratchanavalli07 | 17 Sep, 2024, 07:46: AM
JEE main - Physics
Asked by yayashvadutta45 | 15 Sep, 2024, 19:47: PM
JEE main - Physics
Asked by adithireddy999 | 03 Sep, 2024, 09:35: AM
JEE main - Physics
Asked by vaishalinirmal739 | 29 Aug, 2024, 18:07: PM
JEE main - Physics
Asked by vradhysyam | 26 Aug, 2024, 17:17: PM