JEE Class main Answered
solve
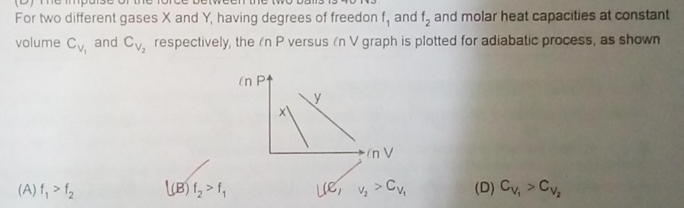
Asked by adjacentcalliber10 | 10 May, 2019, 12:00: PM
for adiabatic process, we have PV γ = K ...................(1)
where P is pressure, V is volume, γ is ratio of specific heat of constant volume and constant pressure and K is a constant.
by taking logarithm on both sides of eqn.(1), we have ln(P) + γ ln(V) = ln(K)
Hence we have, ln(P) = ln(K) - γ ln(V) ..............................(2)
Eqn.(2) shows the linear relationship between ln(P) and ln(V) as shown in graphs of figure given in question with negative slope.
Hence slope of each graph equals the ratio of specific heat of constant volume and constant pressure.
slope of graph-X that represent gas-1 is greater than slope of graph-Y that represent gas-2.
Hence ratio of specific heats of gas-1 is graeter than that of gas-2
γ1 > γ2 ;

Specific heat is directly prportional to degrees of freedom f . Since we have CV2 > CV1 , then f2 > f1
Answered by Thiyagarajan K | 12 May, 2019, 17:24: PM
Application Videos
Concept Videos
JEE main - Physics
Asked by sumalathamadarapu9 | 23 Oct, 2024, 22:06: PM
JEE main - Physics
Asked by py309649 | 13 Oct, 2024, 13:39: PM
JEE main - Physics
Asked by coolskrish | 13 Oct, 2024, 12:50: PM
JEE main - Physics
Asked by midnightmoon3355 | 09 Oct, 2024, 09:09: AM
JEE main - Physics
Asked by rambabunaidu4455 | 03 Oct, 2024, 16:03: PM
JEE main - Physics
Asked by ratchanavalli07 | 17 Sep, 2024, 07:46: AM
JEE main - Physics
Asked by yayashvadutta45 | 15 Sep, 2024, 19:47: PM
JEE main - Physics
Asked by adithireddy999 | 03 Sep, 2024, 09:35: AM
JEE main - Physics
Asked by vaishalinirmal739 | 29 Aug, 2024, 18:07: PM
JEE main - Physics
Asked by vradhysyam | 26 Aug, 2024, 17:17: PM