JEE Class main Answered
Sir, pls solve the following.

Asked by rsudipto | 25 Dec, 2018, 10:02: AM
n = 4 (valence shell); l = 1(p subshell); s =-1/2
following combination cannot be done for the above quantum numbers
| +1 | 0 | -1 |
| ↑↓ | ↑↓ | ↑↓ |
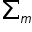 = 2x(+1) + (-1) +0 = +1
= 2x(+1) + (-1) +0 = +1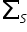 ≠ -1/2
≠ -1/2configuration = 1s2 2s2 sp6 3s2 3p6 4s2 3d10 4p6
adding electrons = 36 (atomic number)
Answered by Ramandeep | 04 Jan, 2019, 10:50: AM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM













